1.2 Chemical Reactions: Ionic Bonding
|
|
Atoms can react with each other to form compounds. When atoms react, they form chemical bonds. Making bonds involves giving, taking or sharing electrons. There are two main types of bonding that are needed for the exam; they are ionic bonding and covalent bonding.
Ionic Bonding
Ionic bonding is where one atom gives an electron to another atom. This results in the two atoms becoming ions because they do not have a neutral charge. The atom who gives the electron away has a positive charge because there are less electrons than protons (because protons have a positive charge and electrons have a negative charge), and the atom who gains an electron becomes negatively charged because the atom now has more electrons than it does protons.
Let’s suppose that we have a compound that is formed from a non-metal and a metal, where both the metal and the non-metal are ions. The metal in this reaction gives electrons to the non-metal. The metal becomes a positive ion, as it now has more protons than electrons. The non-metal gains electrons and therefore becomes a negative ion because there are now more electrons than there are protons. The two ions are attracted to each other because of their opposite charges.
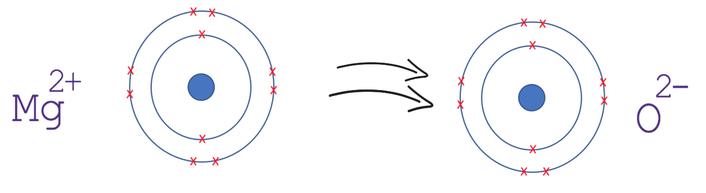
An example is magnesium oxide. Here magnesium gives oxygen 2 electrons. The formula of magnesium oxide is MgO, with magnesium being Mg2+ and oxygen being O2-. Note: we write the charges of atoms as a superscript after the element with the charge sign afterwards. We also draw the atoms with brackets and the charge signalled on the outside.
Ionic bonding is where one atom gives an electron to another atom. This results in the two atoms becoming ions because they do not have a neutral charge. The atom who gives the electron away has a positive charge because there are less electrons than protons (because protons have a positive charge and electrons have a negative charge), and the atom who gains an electron becomes negatively charged because the atom now has more electrons than it does protons.
Let’s suppose that we have a compound that is formed from a non-metal and a metal, where both the metal and the non-metal are ions. The metal in this reaction gives electrons to the non-metal. The metal becomes a positive ion, as it now has more protons than electrons. The non-metal gains electrons and therefore becomes a negative ion because there are now more electrons than there are protons. The two ions are attracted to each other because of their opposite charges.
An example is magnesium oxide. Here magnesium gives oxygen 2 electrons. The formula of magnesium oxide is MgO, with magnesium being Mg2+ and oxygen being O2-. Note: we write the charges of atoms as a superscript after the element with the charge sign afterwards. We also draw the atoms with brackets and the charge signalled on the outside.