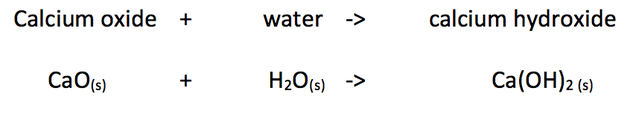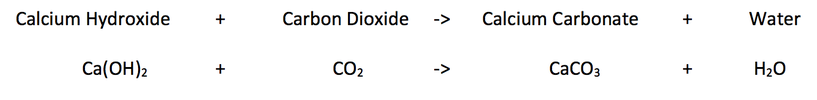2.1 Limestone (part 1)
|
|
Limestone is mainly composed of calcium carbonate (CaCO3). It is naturally occurring and can be quarried out of the ground and used in building materials. Many older buildings, such as churches, are made from limestone. Calcium carbonate has the chemical formula CaCO3, meaning that each molecule comprises of one calcium atom, one carbon atom and three oxygen atoms.
Thermal Decomposition
Calcium carbonate thermal decomposes when it has been heated. When heated it makes calcium oxide and carbon dioxide.
Calcium carbonate thermal decomposes when it has been heated. When heated it makes calcium oxide and carbon dioxide.
Magnesium, copper, zinc and sodium carbonates thermal decompose in the same way when they are heated. For example, copper carbonate -> copper oxide + carbon dioxide (CuCO3 -> CuO + CO2).
Reacting with Water
Calcium oxide reacts with water to produce calcium hydroxide.
Calcium oxide reacts with water to produce calcium hydroxide.
Calcium hydroxide is an alkali and can be used to neutralise acids. It is used in farming to neutralise acidic soil. Calcium hydroxide is used because of its speed to neutralise the soil, but powered calcium carbonate could be used instead but it would take longer for the soil to be neutralised.
Calcium hydroxide can also be used to test for carbon dioxide. If you make limewater (which is calcium hydroxide dissolved into water) and pump a gas through it, the lime water will turn cloudy if the gas contains carbon dioxide. The cloudiness in the water is caused by the calcium hydroxide reacting with the carbon dioxide to form calcium carbonate. Water is also produced when calcium hydroxide and carbon dioxide react.
Calcium hydroxide can also be used to test for carbon dioxide. If you make limewater (which is calcium hydroxide dissolved into water) and pump a gas through it, the lime water will turn cloudy if the gas contains carbon dioxide. The cloudiness in the water is caused by the calcium hydroxide reacting with the carbon dioxide to form calcium carbonate. Water is also produced when calcium hydroxide and carbon dioxide react.