4.1 Crude Oil
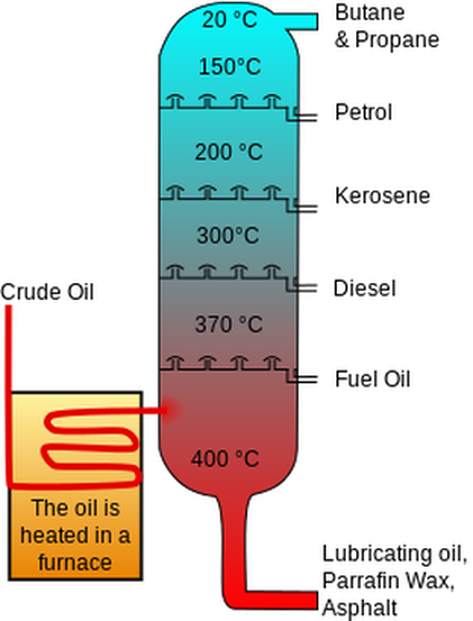 Fractional distillation of crude oil.
Fractional distillation of crude oil.
Crude oil is a fossil fuel that is non-renewable. It has been formed over millions of years by the remnants of plants and animals. Crude oil is extracted by drilling underground. It is a mixture of hydrocarbons. A mixture consists of two or more elements or compounds that are not chemically combined together. Crude oil is a mixture that mainly comprises of hydrocarbons. A hydrocarbon is made up of hydrogen and carbon atoms. They are essentially fuels that we burn to release energy.
The chemical properties of each compound or element in the mixture are unchanged. For example, the compound’s or element’s boiling point remains the same when they are in a mixture as they would be if they were separate. We can therefore separate crude oil into its separate hydrocarbons by heating crude oil up. This process is known as fractional distillation.
Heated crude oil is pumped into a chamber. The chamber is the hottest at the bottom and the coolest at the top. As crude oil is heated, most of the hydrocarbons turn into a gas and therefore rise up in the chamber. The solids, such as bitumen, are not heated up enough to turn into a gas and remain a solid. Bitumen is allowed to come out of the bottom of the chamber. All of the other fuels rise up the chamber because they are a gas. Each hydrocarbon has a different boiling point. As the hydrocarbons rise, each level has different temperatures, which results in a different hydrocarbon condensing at each level. For example, the first level has a temperature of around 340°C and at this temperature oil turns into a liquid. At 340°C all of the other hydrocarbons are still a gas and rise up to another level of the chamber. The next level is at a temperature of 250°C and at this temperature diesel turns into a liquid and all of the other hydrocarbons rise further up (except the ones that have already turned into a liquid (oil) or never turn into a gas (bitumen)). There are more levels in the chamber. The top of the chamber has refined gas, which is bottled gas that we use in BBQs. The longer the hydrocarbon is, the greater the boiling point is. Therefore, the longer hydrocarbons are nearer the bottom and the shorter hydrocarbons are nearer the top of the chamber.
You do not need to know the names, lengths or condensing temperatures for the examination. You only need to know the general idea of what fractional distillation is.
The chemical properties of each compound or element in the mixture are unchanged. For example, the compound’s or element’s boiling point remains the same when they are in a mixture as they would be if they were separate. We can therefore separate crude oil into its separate hydrocarbons by heating crude oil up. This process is known as fractional distillation.
Heated crude oil is pumped into a chamber. The chamber is the hottest at the bottom and the coolest at the top. As crude oil is heated, most of the hydrocarbons turn into a gas and therefore rise up in the chamber. The solids, such as bitumen, are not heated up enough to turn into a gas and remain a solid. Bitumen is allowed to come out of the bottom of the chamber. All of the other fuels rise up the chamber because they are a gas. Each hydrocarbon has a different boiling point. As the hydrocarbons rise, each level has different temperatures, which results in a different hydrocarbon condensing at each level. For example, the first level has a temperature of around 340°C and at this temperature oil turns into a liquid. At 340°C all of the other hydrocarbons are still a gas and rise up to another level of the chamber. The next level is at a temperature of 250°C and at this temperature diesel turns into a liquid and all of the other hydrocarbons rise further up (except the ones that have already turned into a liquid (oil) or never turn into a gas (bitumen)). There are more levels in the chamber. The top of the chamber has refined gas, which is bottled gas that we use in BBQs. The longer the hydrocarbon is, the greater the boiling point is. Therefore, the longer hydrocarbons are nearer the bottom and the shorter hydrocarbons are nearer the top of the chamber.
You do not need to know the names, lengths or condensing temperatures for the examination. You only need to know the general idea of what fractional distillation is.
