1.1 Kinetic Particle theory & states
|
|
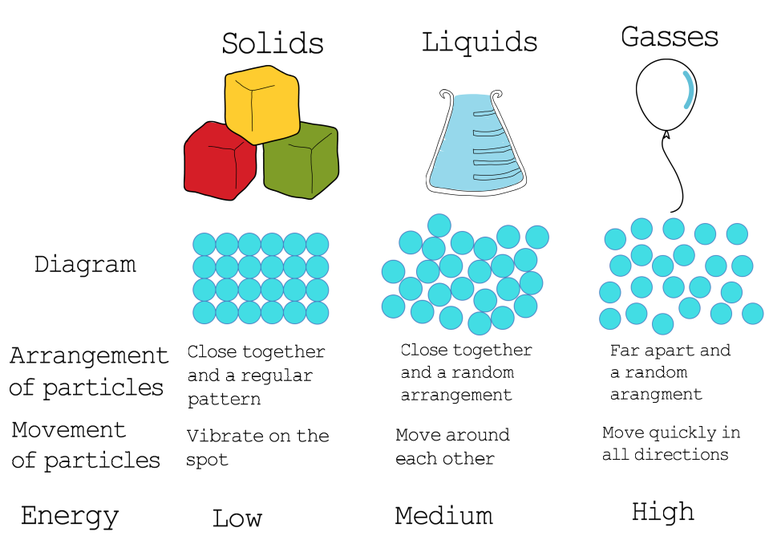
Kinetic Particle Theory explains the properties of the different states of matter. There are 3 states of matter; solids, liquids and gases and each state has different amounts of energy. However, the particles will be the same particles in each of the 3 states. For example, water is still H2O if it is ice (solid), water (liquid) or water vapour (gas).
- Solids: these have a fixed shape and cannot flow. This is because the particles are close together and therefore have no space to move. They can only vibrate in their fixed position and the particles are arranged in a regular pattern.
- Liquids: there are weaker forces of attraction between the particles. The particles can move past each other and flow. The particles will take the shape of their container. They cannot be compressed or squashed. The particles in liquids have more energy than in solids and are also able to move in random directions and at low speeds.
- Gases: the particles in gases are far apart and are in a random arrangement. They move quickly in all directions. A gas will also completely fill its container and can be compressed or squashed. Particles in a gas state have more energy than when they are in the other two states.
The particles will have the most energy when they are in a gas form. They will have the least energy in a solid. The transition from a solid to a liquid is known a liquefaction. This will occur when the particles in a solid are given more energy. A change in state from a liquid to a solid is known as solidification. The process of a liquid gaining energy and turning into a gas is known as evaporation. When a gas turns into a liquid, we observe condensation and this is where the particles lose energy.
Condensation and Evaporation
Evaporation and condensation are changes of states. Evaporation is a liquid becoming a gas and condensation is a gas becoming a liquid.
Evaporation
Evaporation is when a liquid becomes a gas. Some particles in a liquid will have enough kinetic energy to escape the attractive forces from the other particles and can become a gas. The more kinetic energy that a particle has the greater the chance of that particle evaporating. The remaining particles in the liquid have a lower average kinetic energy, which causes the temperature of the liquid to cool. Particles can evaporate at a temperature that is much lower than the boiling point of the liquid.
Sweating is an example of where the cooling effect is useful. As you sweat, some water particles evaporate, which reduces the temperature of the body, which then cools you down even more.
The rate of evaporation depends on many factors. The following factors will increase evaporation:
Condensation
Condensation is when a gas becomes a liquid. As a gas cools, particles lose their kinetic energy. The particles can lose so much of their kinetic energy that the attractive forces between particles bring them closer together and the particles do not have enough kinetic energy to escape. As the particles get brought closer together, bonds form between them. If the particles cool down a considerable amount, the gas will become a liquid.
When you boil a kettle, the steam that comes out of the top of the kettle is water that is condensing as it is coming into cooler air.
The rate of condensation is also dependant on many factors. The following factors will increase condensation:
In addition to condensation and evaporation, we have melting and freezing. Melting is whereby a solid becomes a liquid. Freezing is whereby a liquid becomes a solid.
Also, if we change the pressure of a gas, it will also liquefy. This is because the particles are close enough together for bonds to form between the particles. As soon as the pressure is released, the particles will turn into a gas. This is what happens to gas cylinders used for barbecues. While the gas is in the cylinder, it is a liquid and when the gas is released, it becomes a gas.
Evaporation and condensation are changes of states. Evaporation is a liquid becoming a gas and condensation is a gas becoming a liquid.
Evaporation
Evaporation is when a liquid becomes a gas. Some particles in a liquid will have enough kinetic energy to escape the attractive forces from the other particles and can become a gas. The more kinetic energy that a particle has the greater the chance of that particle evaporating. The remaining particles in the liquid have a lower average kinetic energy, which causes the temperature of the liquid to cool. Particles can evaporate at a temperature that is much lower than the boiling point of the liquid.
Sweating is an example of where the cooling effect is useful. As you sweat, some water particles evaporate, which reduces the temperature of the body, which then cools you down even more.
The rate of evaporation depends on many factors. The following factors will increase evaporation:
- Temperature is higher - if the temperature is higher, the particles will have more kinetic energy and there is a greater chance of them escaping from the other particles
- Surface area is larger - when there is a larger surface area, more particles are closer to the surface, which increases the chance of particles escaping from the liquid.
- Greater airflow over the liquid - if the concentration of an evaporate substance in the air is just above the non-evaporated substance, the evaporation rate of the substance will be lower because as particles evaporate, they are attracted to evaporated particles and as this happens they form a liquid. A greater airflow moves the air above the liquid, meaning that there are fewer evaporated particles in the air above the liquid.
- Density is lower - if density is lower, there are fewer particles to be attracted to, which means particles are able to escape more easily.
Condensation
Condensation is when a gas becomes a liquid. As a gas cools, particles lose their kinetic energy. The particles can lose so much of their kinetic energy that the attractive forces between particles bring them closer together and the particles do not have enough kinetic energy to escape. As the particles get brought closer together, bonds form between them. If the particles cool down a considerable amount, the gas will become a liquid.
When you boil a kettle, the steam that comes out of the top of the kettle is water that is condensing as it is coming into cooler air.
The rate of condensation is also dependant on many factors. The following factors will increase condensation:
- The temperature of the gas is lower - as the temperature falls, the particles have less kinetic energy and start to attract to one another forming a liquid.
- Airflow is less - if the airflow is less, the concentration of particles will be greater, meaning that there are more particles to attract to and this increases the condensation rate.
- Density is higher - if the density is high, the forces between particles will be high meaning that they are attracted to one another. Fewer particles would have enough kinetic energy to overcome these forces and would therefore form a liquid.
In addition to condensation and evaporation, we have melting and freezing. Melting is whereby a solid becomes a liquid. Freezing is whereby a liquid becomes a solid.
Also, if we change the pressure of a gas, it will also liquefy. This is because the particles are close enough together for bonds to form between the particles. As soon as the pressure is released, the particles will turn into a gas. This is what happens to gas cylinders used for barbecues. While the gas is in the cylinder, it is a liquid and when the gas is released, it becomes a gas.