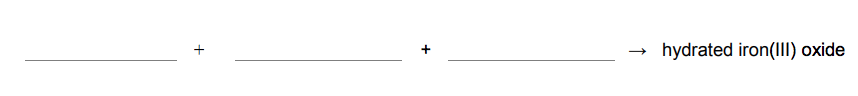Paper 2 H - SAMPLE SET 1 Q3
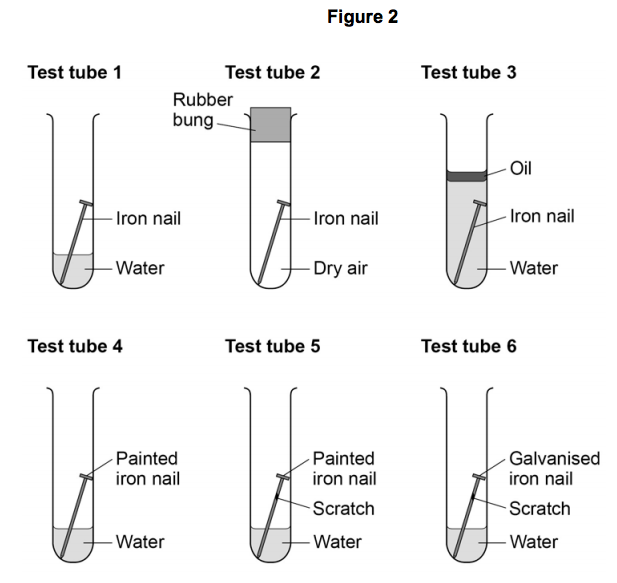
3) Figure 2 shows four test tubes a student set up to investigate the rusting of iron.
This is the method used for each test tube.
This is the method used for each test tube.
- Measure the mass of the nail using a balance.
- Leave the nail in the test tube for 6 days.
- Measure the mass of the nail after 6 days.
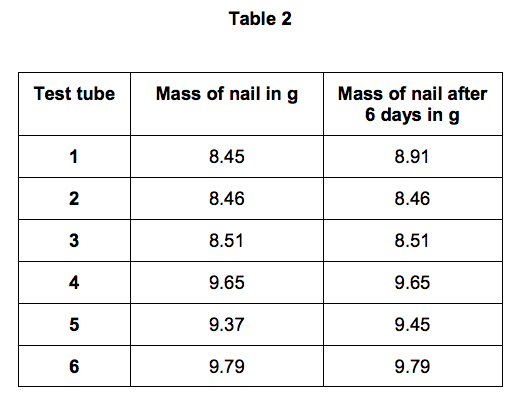
Table 2 shows the student’s measurements.
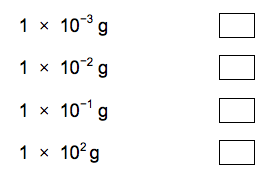
3.1) What is the resolution of the balance the student used? [1 mark]
Tick one box.
Tick one box.
3.2) Calculate the difference in percentage increase in mass after 6 days of the nail in test tube 1 and the nail in test tube 5.
Give your answer to three significant figures. [4 marks]
Give your answer to three significant figures. [4 marks]
Difference in percentage increase in mass = _______________________ %
3.3) Use the results of the student’s investigations to draw conclusions about the factors affecting the rusting of iron. Include an evaluation of the effectiveness of different coatings at preventing the rusting of iron. [6 marks]
3.4) Rust is hydrated iron(III) oxide.
Complete the word equation for the reaction. [2 marks]
3.4) Rust is hydrated iron(III) oxide.
Complete the word equation for the reaction. [2 marks]
(Total for Question 3 = 13 marks)