Paper 2 H - SAMPLE SET 1 Q7
7) A student investigated food dyes using paper chromatography.
This is the method used.
1. Put a spot of food colouring X on the start line.
2. Put spots of four separate dyes, A, B, C and D, on the start line.
3. Place the bottom of the paper in water and leave it for several minutes.
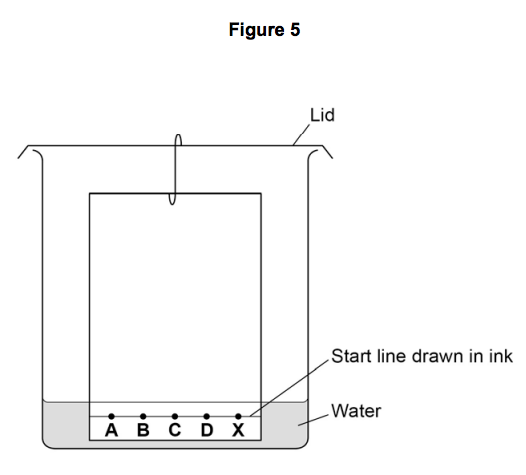
Figure 5 shows the apparatus the student used.
This is the method used.
1. Put a spot of food colouring X on the start line.
2. Put spots of four separate dyes, A, B, C and D, on the start line.
3. Place the bottom of the paper in water and leave it for several minutes.
Figure 5 shows the apparatus the student used.
7.1) Write down two mistakes the student made in setting up the experiment and explain
what problems one of the mistakes would cause.
[2 marks]
Another student set up the apparatus correctly.
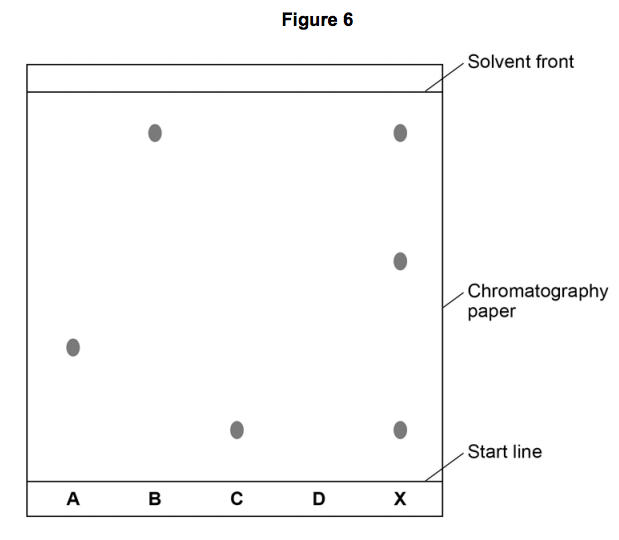
Figure 6 shows the student’s results. The result for dye D is not shown.
Another student set up the apparatus correctly.
Figure 6 shows the student’s results. The result for dye D is not shown.
7.2) Calculate the Rf value of dye A
Give your answer to two significant figures. [3 marks]
Give your answer to two significant figures. [3 marks]
Rf value = ________________________
7.3) Dye D has an Rf value of 0.80. Calculate the distance that dye D moved on the chromatography paper.
[1 mark]
Distance moved by dye D = ________________________
7.4) Explain how the different dyes in X are separated by paper chromatography.
[4 marks]
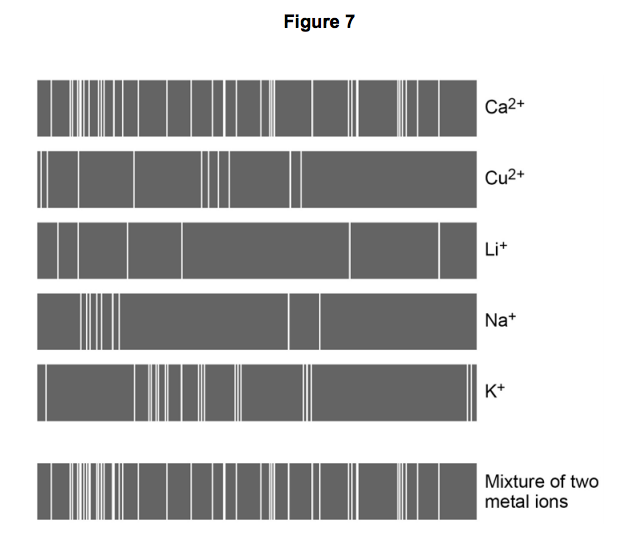
7.5) Flame emission spectroscopy can be used to analyse metal ions in solution.
Figure 7 gives the flame emission spectra of five metal ions, and of a mixture of two metal ions.
Use the spectra to identify the two metal ions in the mixture.
[2 marks]
7.6) Explain why a flame test could not be used to identify the two metal ions in the mixture. [2 marks]
7.7) Two students tested a green compound X.
The students added water to compound X.
Compound X did not dissolve.
The students then added a solution of ethanoic acid to compound X.
A gas was produced which turned limewater milky.
Student A concluded that compound X was sodium carbonate.
Student B concluded that compound X was copper chloride.
Which student, if any, was correct? Explain your reasoning. [4 marks]
(Total for Question 7 = 18 marks)
7.6) Explain why a flame test could not be used to identify the two metal ions in the mixture. [2 marks]
7.7) Two students tested a green compound X.
The students added water to compound X.
Compound X did not dissolve.
The students then added a solution of ethanoic acid to compound X.
A gas was produced which turned limewater milky.
Student A concluded that compound X was sodium carbonate.
Student B concluded that compound X was copper chloride.
Which student, if any, was correct? Explain your reasoning. [4 marks]
(Total for Question 7 = 18 marks)