Paper 2 H - SAMPLE SET 1 Q7
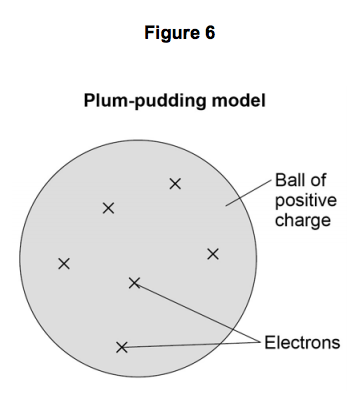
7) Figure 6 shows the plum pudding model of the atom.
This model was used by some scientists after the discovery of electrons in 1897.
This model was used by some scientists after the discovery of electrons in 1897.
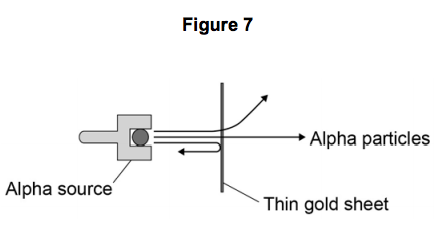
In 1911 the scientists Geiger and Marsden investigated the effect of firing alpha
particles at very thin sheets of gold foil.
Their experiment is shown in Figure 7. The arrows show the paths taken by alpha particles in the experiment.
Their experiment is shown in Figure 7. The arrows show the paths taken by alpha particles in the experiment.
7.1) Explain why scientists replaced the plum pudding model of the atom with the
nuclear model of the atom as a result of the experiment.
[4 marks]
7.2) According to modern measurements:
• the radius of an atom is about 1 × 10–10m
• the radius of an atomic nucleus is about 1 × 10–14m
Show that these values fit with the nuclear model of the atom. [2 marks]
7.3) In 1931 a scientist discovered that there are hydrogen atoms with mass number 2 as well as hydrogen atoms with mass number 1.
A year later, another scientist discovered neutrons.
Explain why the discovery of neutrons could explain the presence of hydrogen atoms with different mass numbers. [3 marks]
7.4) How would the results of the experiment shown in Figure 7 change if neutrons were used instead of alpha particles to bombard a thin sheet of gold? [2 marks]
(Total for Question 7 = 11 marks)
7.2) According to modern measurements:
• the radius of an atom is about 1 × 10–10m
• the radius of an atomic nucleus is about 1 × 10–14m
Show that these values fit with the nuclear model of the atom. [2 marks]
7.3) In 1931 a scientist discovered that there are hydrogen atoms with mass number 2 as well as hydrogen atoms with mass number 1.
A year later, another scientist discovered neutrons.
Explain why the discovery of neutrons could explain the presence of hydrogen atoms with different mass numbers. [3 marks]
7.4) How would the results of the experiment shown in Figure 7 change if neutrons were used instead of alpha particles to bombard a thin sheet of gold? [2 marks]
(Total for Question 7 = 11 marks)