Paper 4 H - SAMPLE SET 1 Q2 Answers
2) A student investigates how the concentration of an acid affects the rate of a reaction.
This is the method used.
1. Put a 3 cm piece of magnesium ribbon into a conical flask.
2. Add 50 cm3 of 0.5 mol/dm3 hydrochloric acid to the flask.
3. Collect and measure the volume of gas produced at 10 second intervals.
4. Repeat with different concentrations of hydrochloric acid using the same length of magnesium ribbon and volume of acid.
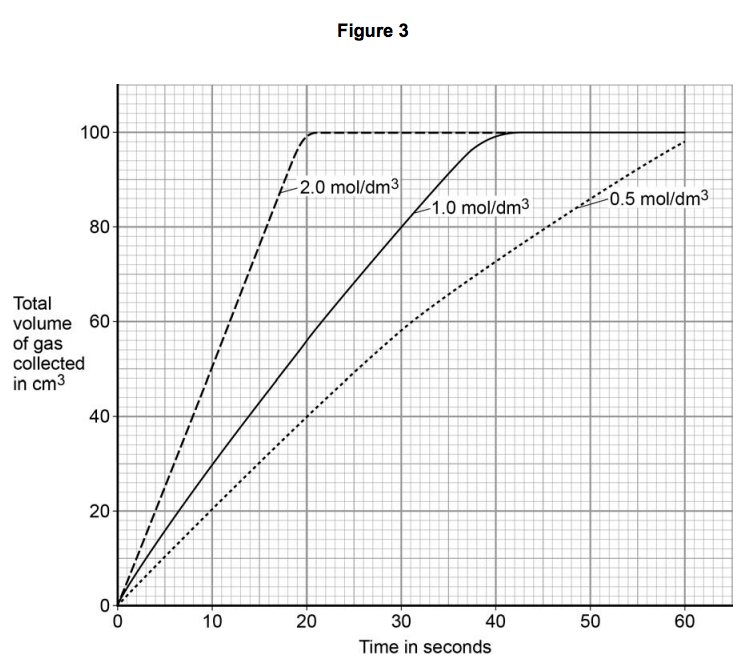
The student’s results are shown in Figure 3.
This is the method used.
1. Put a 3 cm piece of magnesium ribbon into a conical flask.
2. Add 50 cm3 of 0.5 mol/dm3 hydrochloric acid to the flask.
3. Collect and measure the volume of gas produced at 10 second intervals.
4. Repeat with different concentrations of hydrochloric acid using the same length of magnesium ribbon and volume of acid.
The student’s results are shown in Figure 3.
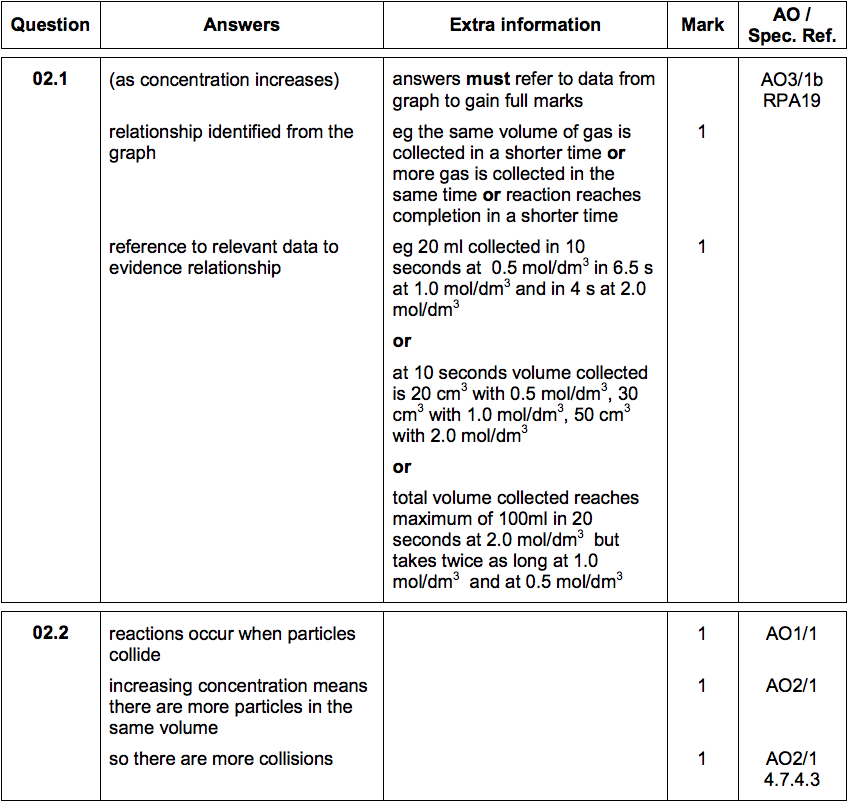
2.1) How do the results show that increasing the concentration of acid increases the rate of reaction?
[2 marks]
2.2) Explain why the rate of reaction changes as the concentration of the acid increases.
You should answer in terms of particles. [3 marks]
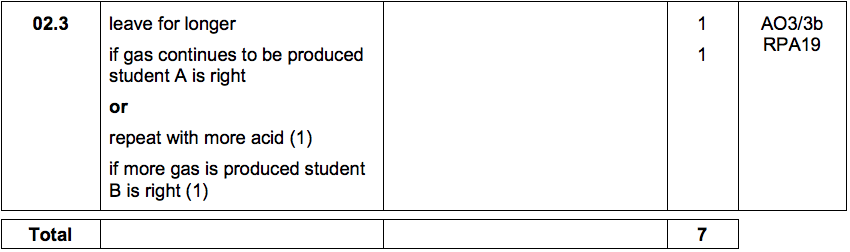
2.3) Student A said that the final volume of gas collected was lower for a concentration of 0.5 mol dm3 because the reaction had not finished.
Student B said it was because all the acid had reacted.
Describe further experimental work the students could do to find out which student was correct. [2 marks]
(Total for Question 2 = 7 marks)
2.2) Explain why the rate of reaction changes as the concentration of the acid increases.
You should answer in terms of particles. [3 marks]
2.3) Student A said that the final volume of gas collected was lower for a concentration of 0.5 mol dm3 because the reaction had not finished.
Student B said it was because all the acid had reacted.
Describe further experimental work the students could do to find out which student was correct. [2 marks]
(Total for Question 2 = 7 marks)