Q2 Answers
Questions
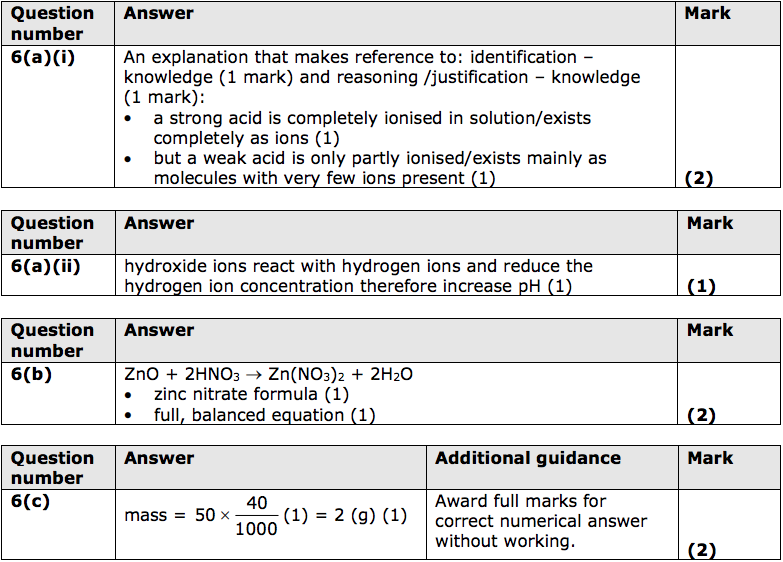
6) Some acids such as hydrochloric acid are described as strong acids.
Some acids such as ethanoic acid are described as weak acids.
(a) (i) Explain the difference between a strong acid and a weak acid. (2)
(ii) Give a reason why adding hydroxide ions to an acid solution leads to an increase in pH. (1)
(b) The salt zinc nitrate can be made by reacting zinc oxide, ZnO, with dilute nitric acid, HNO3.
Write the balanced equation for this reaction. (2)
(c) 50cm3 of potassium hydroxide solution of concentration 40g dm–3 is needed for an experiment.
Calculate the mass of potassium hydroxide that must be dissolved in water to make 50cm3 of solution of this concentration. (2)
Some acids such as ethanoic acid are described as weak acids.
(a) (i) Explain the difference between a strong acid and a weak acid. (2)
(ii) Give a reason why adding hydroxide ions to an acid solution leads to an increase in pH. (1)
(b) The salt zinc nitrate can be made by reacting zinc oxide, ZnO, with dilute nitric acid, HNO3.
Write the balanced equation for this reaction. (2)
(c) 50cm3 of potassium hydroxide solution of concentration 40g dm–3 is needed for an experiment.
Calculate the mass of potassium hydroxide that must be dissolved in water to make 50cm3 of solution of this concentration. (2)
mass of potassium hydroxide = ..................................................................... g
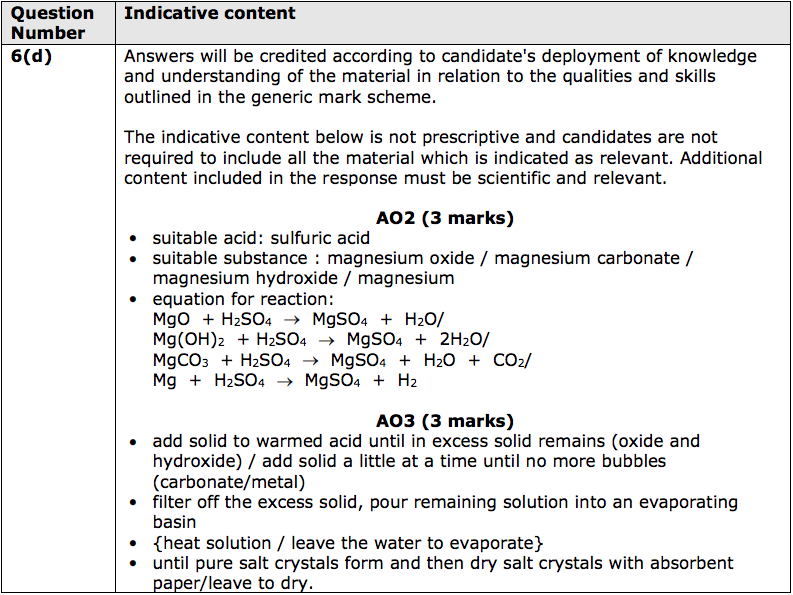
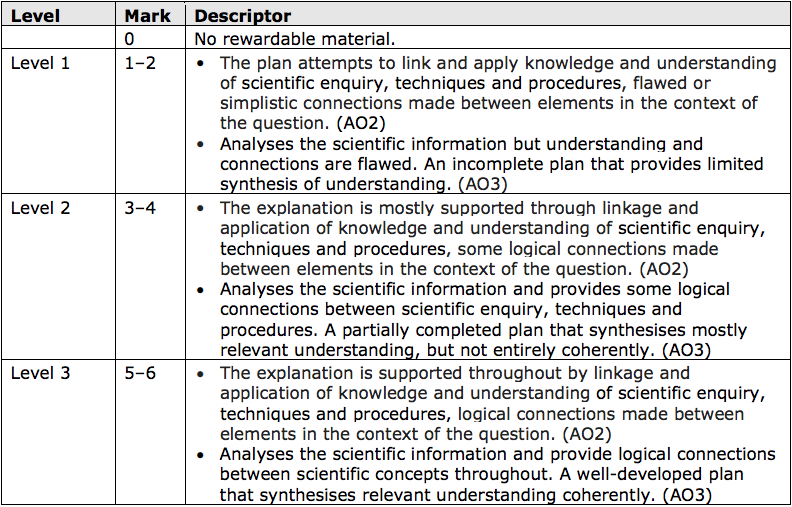
*(d) Salts of metals can be made by reacting one of the metal’s compounds with the
appropriate acid.
Plan an experiment to prepare pure, dry crystals of magnesium sulfate, MgSO4 , by reacting a suitable magnesium compound with a suitable acid.
You may use equations if you wish. (6)
(Total for Question 6 = 13 marks)
Plan an experiment to prepare pure, dry crystals of magnesium sulfate, MgSO4 , by reacting a suitable magnesium compound with a suitable acid.
You may use equations if you wish. (6)
(Total for Question 6 = 13 marks)