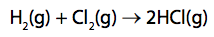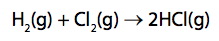Q2
6) There is no part a in this question.
(b) When sodium hydroxide solution is neutralised with an acid there is a temperature change.
A student is given dilute hydrochloric acid and dilute ethanoic acid of the same concentration in mol dm−3.
Devise a plan to compare the temperature changes produced when sodium hydroxide solution is neutralised with each of these two acids. (4)
(c) Hydrogen reacts with chlorine to form hydrogen chloride.
(b) When sodium hydroxide solution is neutralised with an acid there is a temperature change.
A student is given dilute hydrochloric acid and dilute ethanoic acid of the same concentration in mol dm−3.
Devise a plan to compare the temperature changes produced when sodium hydroxide solution is neutralised with each of these two acids. (4)
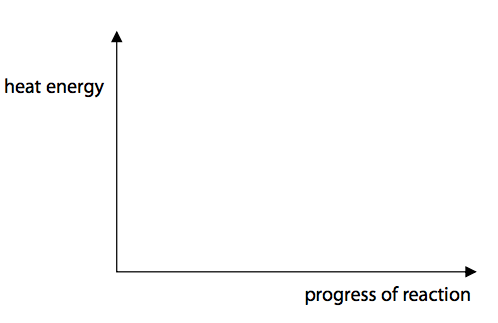
(c) Hydrogen reacts with chlorine to form hydrogen chloride.
The reaction is exothermic.
Draw and label the reaction profile diagram for this reaction, identifying the activation energy. (3)
Draw and label the reaction profile diagram for this reaction, identifying the activation energy. (3)
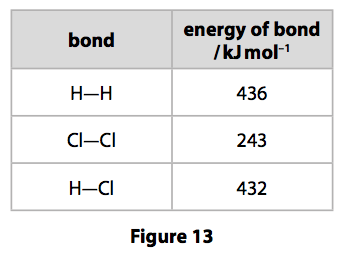
(d) The energies of some bonds are shown in Figure 13.
Hydrogen reacts with chlorine to form hydrogen chloride.
Calculate the energy change, in kJ mol–1, for the reaction of 1mol of hydrogen gas, H2, with 1 mol of chlorine gas, Cl2, to form 2mol of hydrogen chloride gas, HCl. (4)
energy change = .............................................................. kJ mol−1
(Total for Question 6 = 12 marks)