6.1 - SPECIMEN SET C2 Q3 Answers
3) A student investigated the rate of the reaction between magnesium and dilute hydrochloric acid.
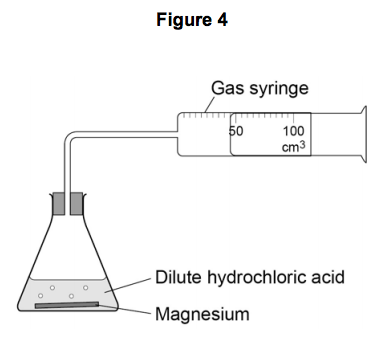
The student used the apparatus shown in Figure 4 to collect the gas produced.
The student used the apparatus shown in Figure 4 to collect the gas produced.
3.1) Outline a plan to investigate how the rate of this reaction changed when the concentration of the hydrochloric acid was changed.
- Describe how you would do the investigation and the measurements you would make.
- Describe how you would make it a fair test.
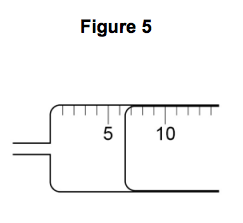
3.2) Figure 5 shows the gas syringe during one of the experiments.
What is the volume of gas collected? [1 mark]
Tick one box.
What is the volume of gas collected? [1 mark]
Tick one box.
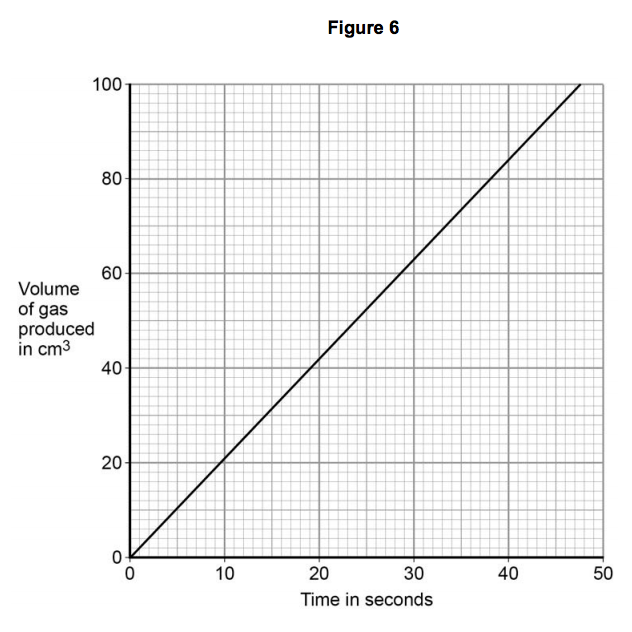
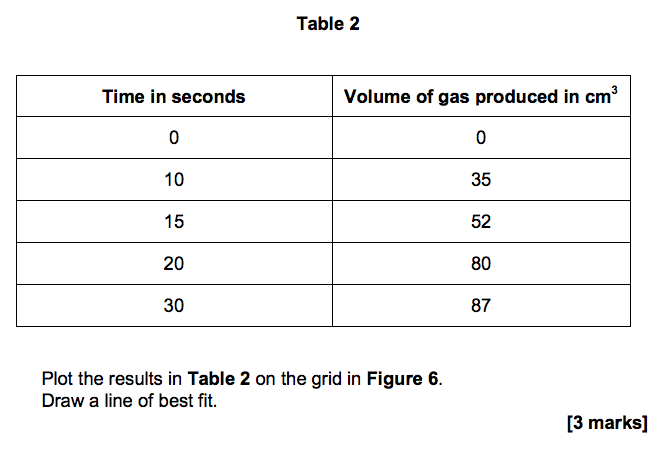
3.3) Figure 6 shows the student’s results for one concentration of hydrochloric acid.
Table 2 shows the student’s results when the concentration was two times greater than the results on Figure 6.
Plot the results in Table 2 on the grid in Figure 6. Draw a line of best fit. [3 marks]
3.4) Give one conclusion about how the rate of reaction changed when the concentration of hydrochloric acid was changed. [1 mark]
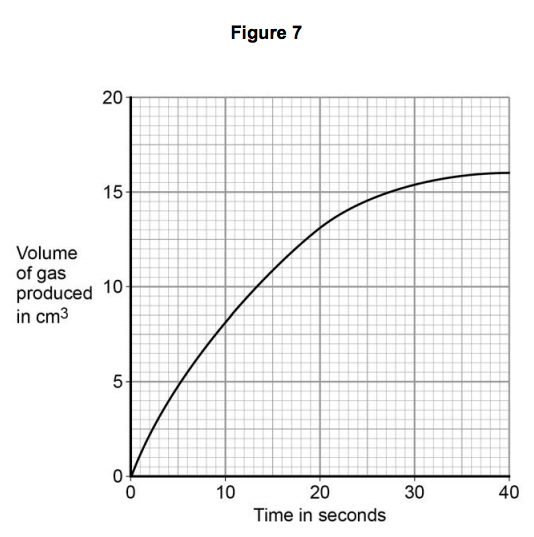
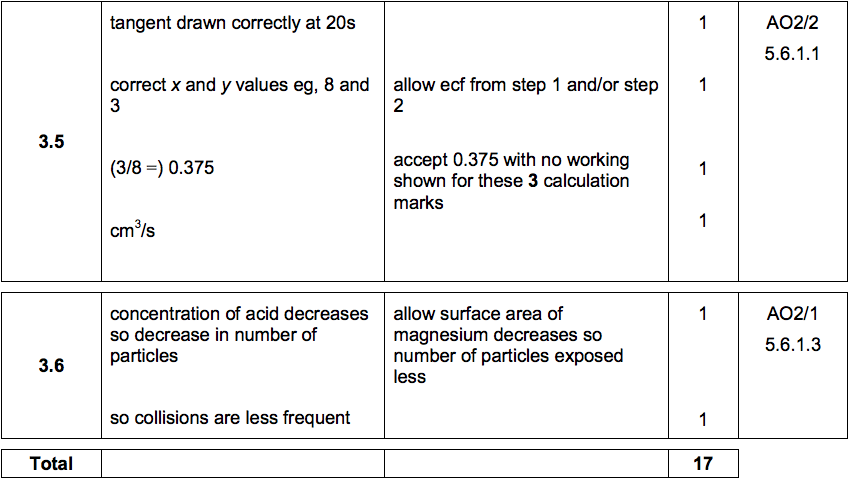
3.5) Figure 7 shows volume of gas produced against time for the reaction between magnesium and ethanoic acid.
3.4) Give one conclusion about how the rate of reaction changed when the concentration of hydrochloric acid was changed. [1 mark]
3.5) Figure 7 shows volume of gas produced against time for the reaction between magnesium and ethanoic acid.
Draw a tangent to the curve at 20 seconds.
Determine the rate of the reaction at 20 seconds by calculating the gradient of the tangent.
Give the unit. [4 marks]
Rate of reaction = ________________________
Unit = _______________________________
3.6) Explain, in terms of particles, why the rate decreases during the reaction between magnesium and ethanoic acid. [2 marks]
(Total for Question 3 = 17 marks)
Determine the rate of the reaction at 20 seconds by calculating the gradient of the tangent.
Give the unit. [4 marks]
Rate of reaction = ________________________
Unit = _______________________________
3.6) Explain, in terms of particles, why the rate decreases during the reaction between magnesium and ethanoic acid. [2 marks]
(Total for Question 3 = 17 marks)