Paper 1 H - SAMPLE SET 1 Q6 Answers
Questions
6) The particle model can be used to explain the properties of gases.
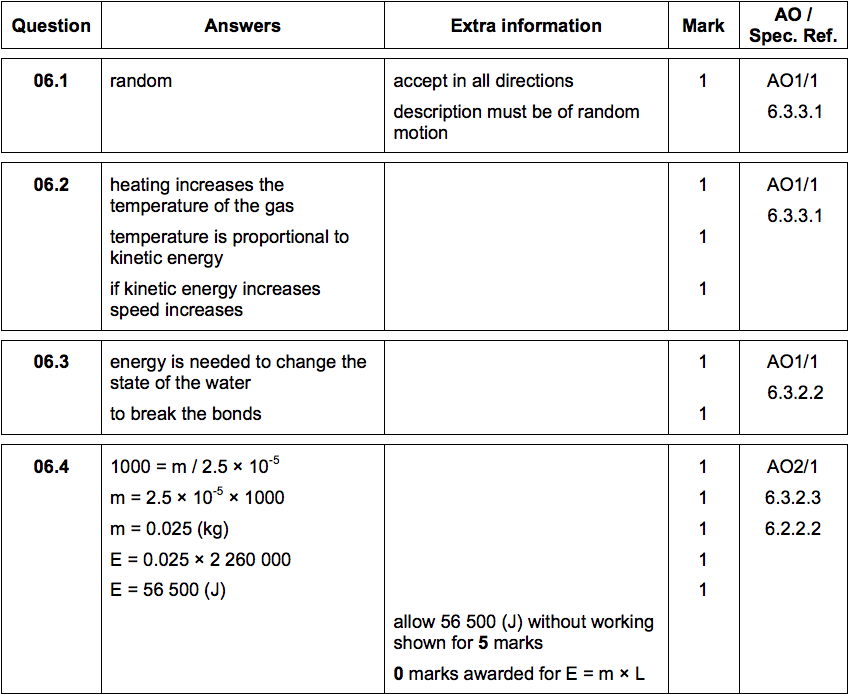
6.1)Describe the direction of motion of the particles in a gas [1 mark]
6.2) Explain why heating a gas increases the average speed of the gas particles. [3 marks]
6.3) Water can exist as either a liquid or a gas at 100 °C.
Explain why a mass of gaseous water at 100 °C contains more energy than an equal mass of liquid water at 100 °C. [2 marks]
Water vapour is a gas. Gases change state when they cool.
Figure 8 shows condensation on a cold bathroom mirror.
6.1)Describe the direction of motion of the particles in a gas [1 mark]
6.2) Explain why heating a gas increases the average speed of the gas particles. [3 marks]
6.3) Water can exist as either a liquid or a gas at 100 °C.
Explain why a mass of gaseous water at 100 °C contains more energy than an equal mass of liquid water at 100 °C. [2 marks]
Water vapour is a gas. Gases change state when they cool.
Figure 8 shows condensation on a cold bathroom mirror.
6.4) A volume of 2.5 × 10–5m3
of condensation forms on the mirror.
Density of water = 1000 kg/m3
Specific latent heat of vaporisation of water = 2.26 x 106 J/kg.
Calculate the energy released when the condensation forms. [5 marks]
Density of water = 1000 kg/m3
Specific latent heat of vaporisation of water = 2.26 x 106 J/kg.
Calculate the energy released when the condensation forms. [5 marks]
Energy released = _____________ J
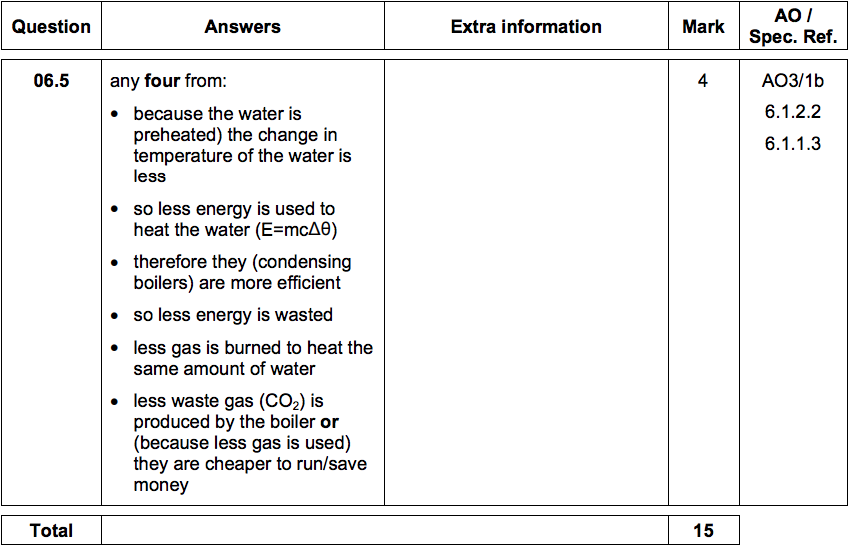
6.5) Central heating boilers burn gas and use the energy released to heat water.
Modern condensing central heating boilers take advantage of the energy that is
released when water condenses.
Waste water vapour produced when the water is heated in the boiler is used to
preheat the cold water entering the boiler.
Give some of the arguments in favour of condensing boilers compared to older
non-condensing boilers.
[4 marks]
(Total for Question 6 = 15 marks)