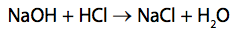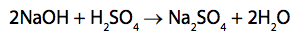C1 H - Sample set 1 Q10
10) (a) In an experiment, ammonia gas is made by heating a mixture of ammonium
chloride and calcium hydroxide.
10.0g of ammonium chloride is added to an excess of calcium hydroxide.
Calculate the maximum volume of ammonia gas that could be formed.
(relative atomic mass H = 1.00, N = 14.0, O = 16.0 and Ca = 40.0; one mole of any gas occupies 24dm3 at room temperature and pressure) (2)
Calculate the maximum volume of ammonia gas that could be formed.
(relative atomic mass H = 1.00, N = 14.0, O = 16.0 and Ca = 40.0; one mole of any gas occupies 24dm3 at room temperature and pressure) (2)
volume = ........................................................................... dm3
(b) Sodium hydroxide solution reacts with hydrochloric acid.
(i) 25.0cm3
of 0.100mol dm-3 sodium hydroxide, NaOH, solution is added to 35.0cm3
of 0.0750mol dm-3 dilute hydrochloric acid, HCl.
Use the information to determine which reagent is in excess. (3)
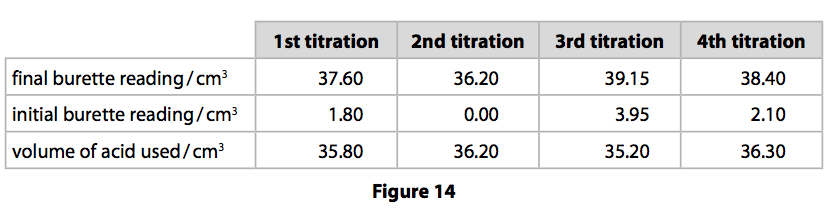
(ii) To find the exact amount of dilute hydrochloric acid that reacts with 25.0cm3 of the sodium hydroxide solution, a titration is carried out. Figure 14 shows the results for the titrations.
Use the information to determine which reagent is in excess. (3)
(ii) To find the exact amount of dilute hydrochloric acid that reacts with 25.0cm3 of the sodium hydroxide solution, a titration is carried out. Figure 14 shows the results for the titrations.
In this titration, the accurate volumes of acid used that are within 0.20cm3 of
each other are considered concordant volumes.
Use the concordant results to calculate the mean volume of hydrochloric acid required. (1)
Use the concordant results to calculate the mean volume of hydrochloric acid required. (1)
mean volume = ....................................................................... cm3
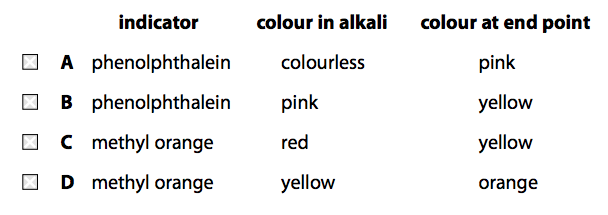
(iii) During the titration, the indicator used changed colour at the end point.
Which of the following shows an indicator with the colour change that would be seen in this titration? (1)
Which of the following shows an indicator with the colour change that would be seen in this titration? (1)
(c) In another titration, 25.0cm3
of a different sodium hydroxide solution is titrated
with 0.200mol dm–3 sulfuric acid, H2SO4
.
24.80cm3
of acid are required to neutralise 25.0 cm3
of the sodium hydroxide solution.
Calculate the concentration of the sodium hydroxide solution, NaOH, in mol dm−3. (4)
Calculate the concentration of the sodium hydroxide solution, NaOH, in mol dm−3. (4)
concentration = ............................................................. mol dm−3
(Total for Question 10 = 11 marks)