C1 H - Sample set 1 Q3 Answers
3) A student carried out an experiment to see how reactive different metals are when they are placed in dilute hydrochloric acid.
A sample of each metal was placed in a separate test tube of acid.
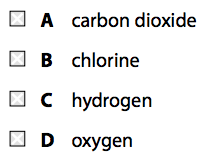
(a) When zinc reacts with dilute hydrochloric acid, a gas is given off and zinc chloride is formed.
(i) Which gas is given off?(1)
A sample of each metal was placed in a separate test tube of acid.
(a) When zinc reacts with dilute hydrochloric acid, a gas is given off and zinc chloride is formed.
(i) Which gas is given off?(1)
(ii) What is the formula of zinc chloride? (1)
(b) In the experiment, the student used the same amount of each metal in a finely powdered form.
State two factors, concerning the hydrochloric acid, which should also be controlled to produce valid results. (2)
(c) Part of the reactivity series is shown in Figure 8.
State two factors, concerning the hydrochloric acid, which should also be controlled to produce valid results. (2)
- ...............................................................................................
- ...............................................................................................
(c) Part of the reactivity series is shown in Figure 8.
Iron is extracted from its ore by heating with carbon.
Aluminium is extracted from its ore using a different method.
(i) Give the name of the method used to extract aluminium. (1)
(ii) Explain why aluminium is extracted by a different method rather than heating the ore with carbon. (2)
(d) The extraction of iron involves the reduction of iron oxide, Fe2O3 , by carbon monoxide, CO. During this reaction, the iron oxide is reduced to iron, Fe, and the carbon monoxide is oxidised to carbon dioxide.
Write the balanced equation for the reaction. (2)
(Total for Question 3 = 9 marks)