Q2
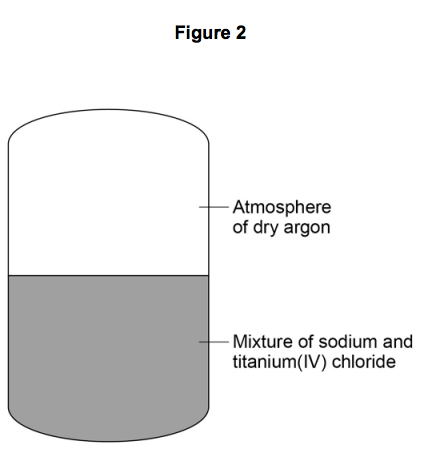
2) Figure 2 shows a reactor used to produce titanium from titanium(IV) chloride.
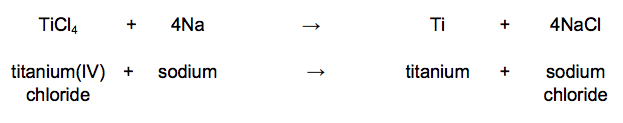
The chemical equation for the reaction of titanium(IV) chloride with sodium is:
For one reaction:
• 1615 kg titanium(IV) chloride reacted completely with 782 kg sodium
• 1989 kg sodium chloride was produced.
Calculate the mass of titanium produced from this reaction. [1 mark]
• 1615 kg titanium(IV) chloride reacted completely with 782 kg sodium
• 1989 kg sodium chloride was produced.
Calculate the mass of titanium produced from this reaction. [1 mark]
Mass of titanium = ________________________ kg
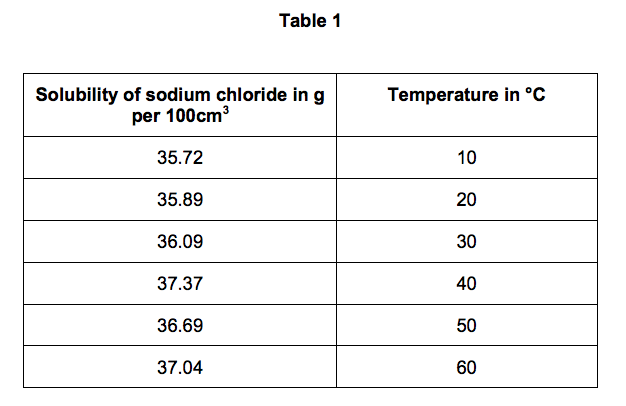
2.2) Table 1 shows the solubility of sodium chloride in 100 cm3 of aqueous solution
at different temperatures.
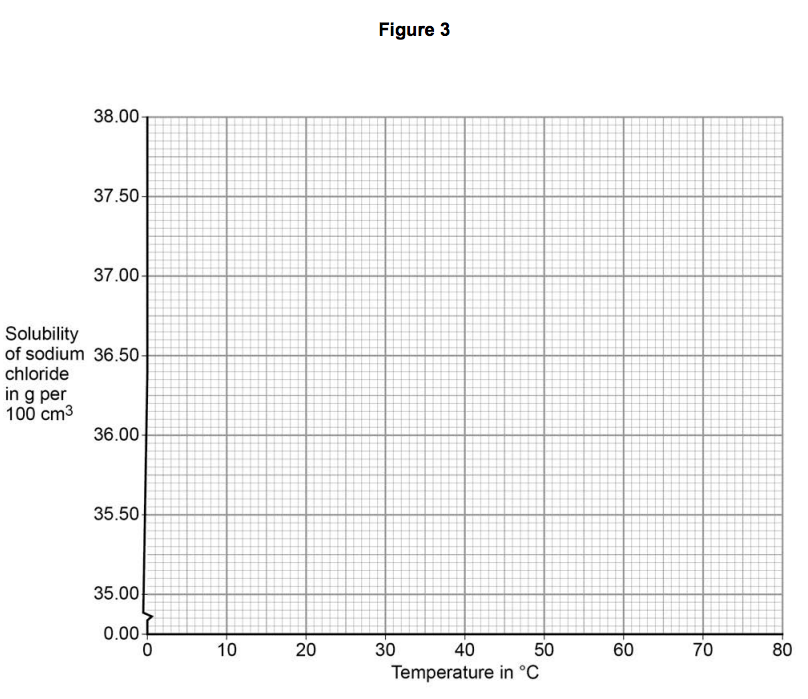
On Figure 3:
• plot this data on the grid
• draw a line of best fit. [3 marks]
• plot this data on the grid
• draw a line of best fit. [3 marks]
2.3) The product sodium chloride is dissolved in water to separate it from
titanium.
At 30 °C the solubility of sodium chloride is 36 kg per 100 dm3.
Calculate the minimum volume of water in dm3, at 30 °C, needed to dissolve 1989 kg sodium chloride. [2 marks]
At 30 °C the solubility of sodium chloride is 36 kg per 100 dm3.
Calculate the minimum volume of water in dm3, at 30 °C, needed to dissolve 1989 kg sodium chloride. [2 marks]
Volume of water = ____________________ dm3
2.4)Calculate the percentage by mass of titanium in titanium(IV) chloride (TiCl4).
Give your answer to 3 significant figures.
Relative atomic masses (Ar): Cl = 35.5; Ti = 48
[3 marks]
Percentage of titanium by mass = _________ %
2.5) Suggest why the reaction is done in an atmosphere of dry argon instead of air
containing water vapour.
[3 marks]
2.6)Explain why titanium conducts electricity.
[3 marks]
(Total for Question 2 = 15 marks)