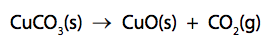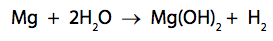Paper 1 SPECIMEN (SET 1) - Q5 Answers
Questions
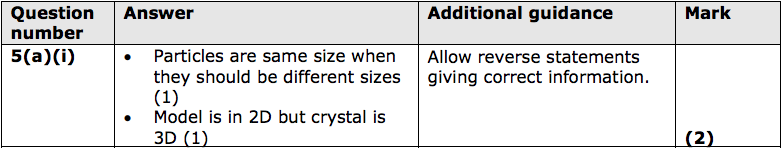
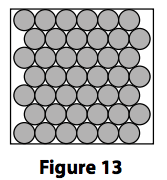
5) Figure 13 shows a model of how particles are arranged in a solid.
(a) (i) State two ways in which this model fails to accurately represent a crystal of
sodium chloride.
(2)
1 ...........................................................................
2 ...........................................................................
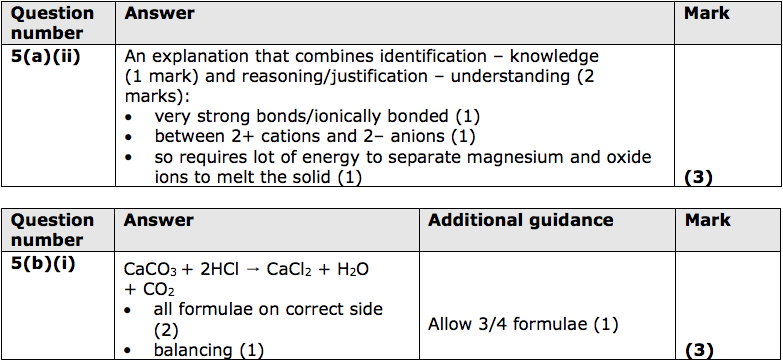
(ii) Magnesium oxide has a melting point of 2852°C.
Explain why magnesium oxide has such a high melting point. (3)
(b) (i) Carbon dioxide can be formed by the reaction of calcium carbonate, CaCO3 , with dilute hydrochloric acid.
Write the balanced equation for this reaction. (3)
(ii) The thermal decomposition of copper carbonate forms copper oxide and carbon dioxide.
1 ...........................................................................
2 ...........................................................................
(ii) Magnesium oxide has a melting point of 2852°C.
Explain why magnesium oxide has such a high melting point. (3)
(b) (i) Carbon dioxide can be formed by the reaction of calcium carbonate, CaCO3 , with dilute hydrochloric acid.
Write the balanced equation for this reaction. (3)
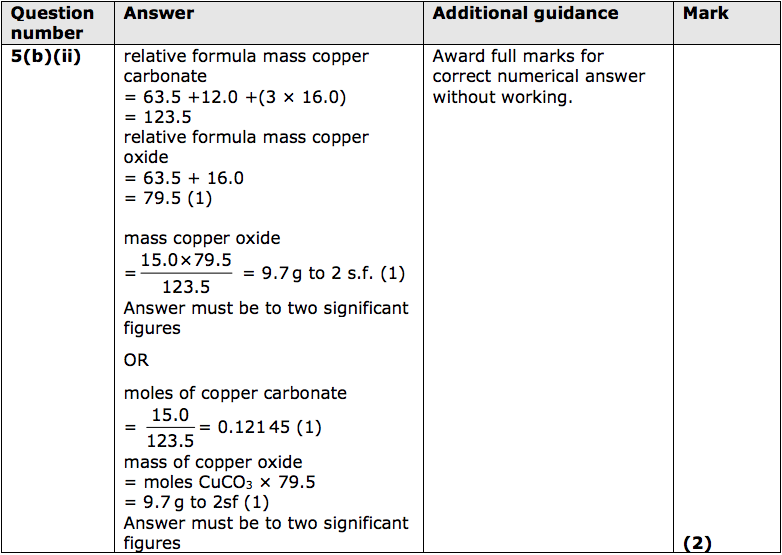
(ii) The thermal decomposition of copper carbonate forms copper oxide and carbon dioxide.
15.0g of pure copper carbonate is decomposed completely.
Calculate the mass of solid produced.
(relative atomic masses: C = 12.0; O = 16.0; Cu = 63.5)
Give your answer to two significant figures. (2)
Calculate the mass of solid produced.
(relative atomic masses: C = 12.0; O = 16.0; Cu = 63.5)
Give your answer to two significant figures. (2)
mass of solid = ........................................... g
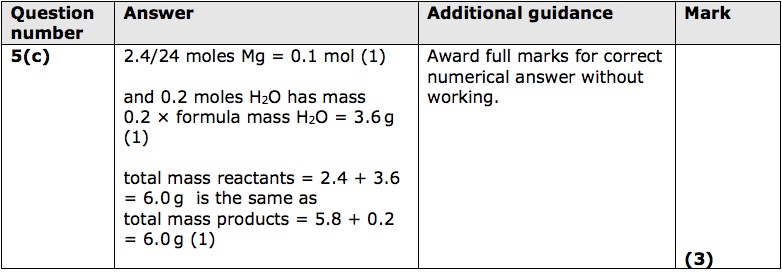
(c) Magnesium reacts with water in the form of steam as shown in the equation.
2.4g of magnesium reacts with sufficient steam for a complete reaction to form
5.8g of magnesium hydroxide and 0.2g of hydrogen.
Show, by calculation, that the law of conservation of mass applies to this reaction.
(relative atomic masses: H = 1.0, O = 16, Mg = 24) (3)
(Total for Question 5 = 13 marks)
Show, by calculation, that the law of conservation of mass applies to this reaction.
(relative atomic masses: H = 1.0, O = 16, Mg = 24) (3)
(Total for Question 5 = 13 marks)