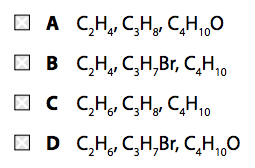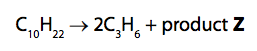Paper 2 SPECIMEN (SET 1) - Q3
3) Crude oil is a mixture of hydrocarbons.
It can be separated into fractions.
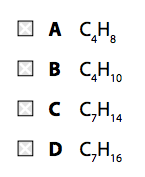
(a) Which of these mixtures shows formulae of substances that could be in the gaseous fraction of crude oil? (1)
It can be separated into fractions.
(a) Which of these mixtures shows formulae of substances that could be in the gaseous fraction of crude oil? (1)
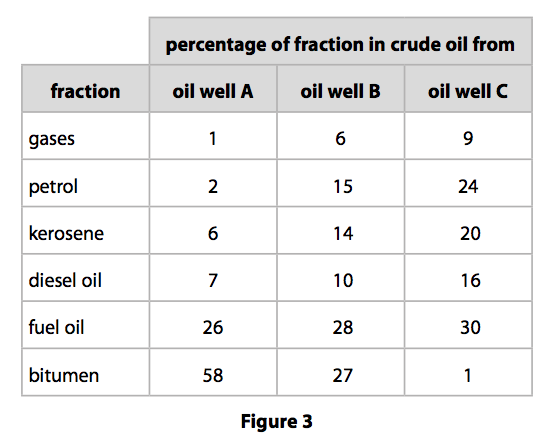
(b) Figure 3 shows the percentages of the fractions in crude oil from three different
oil wells.
(i) State which oil well contains the greatest combined total of diesel oil and
fuel oil.
(1)
(ii) State which oil well produces a crude oil containing the highest percentage of the high boiling point fractions. (1)
(c) Fractions of crude oil contain alkanes.
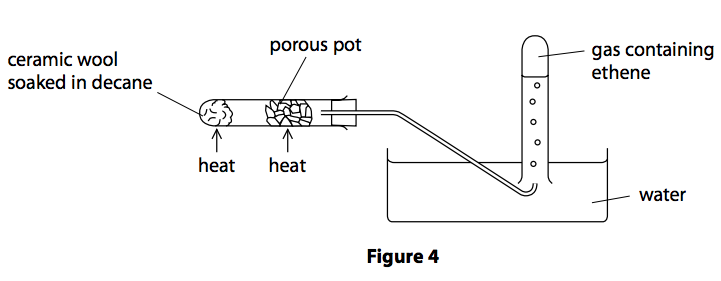
A sample of decane, C10H22, cracked using the apparatus in Figure 4.
(ii) State which oil well produces a crude oil containing the highest percentage of the high boiling point fractions. (1)
(c) Fractions of crude oil contain alkanes.
A sample of decane, C10H22, cracked using the apparatus in Figure 4.
(i) Explain how ethene is produced using the apparatus in Figure 4.
(3)
(ii) One molecule of decane produced two molecules of propene, C3H6, and one molecule of product Z.
(ii) One molecule of decane produced two molecules of propene, C3H6, and one molecule of product Z.
What is the formula of product Z?
(1)
(iii) When decane undergoes complete combustion, a mixture of carbon dioxide
and water is formed.
Complete the balanced equation for this reaction. (2)
Complete the balanced equation for this reaction. (2)
(Total for Question 3 = 9 marks)