Paper 2 SPECIMEN (SET 1) - Q5
5) The elements chlorine, bromine and iodine are part of group 7 in the periodic table.
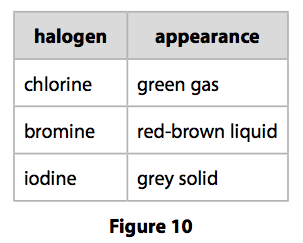
(a) The appearances of chlorine, bromine and iodine at room temperature are shown in Figure 10.
(a) The appearances of chlorine, bromine and iodine at room temperature are shown in Figure 10.
Astatine is the element below iodine in group 7.
Predict the appearance of astatine. (1)
*(b) The order of reactivity of chlorine, bromine and iodine can be determined by carrying out displacement reactions.
Explain how displacement reactions can be used to show the reactivity of these three elements. (6)
(c) When iron wool is heated in bromine vapour, it reacts to form iron bromide.
(i) In an experiment, 5.60g of iron reacted exactly with 24.0g of bromine, Br2.
[relative atomic masses: Fe = 56.0, Br = 80.0]
Determine, using this information, the balanced equation for the reaction between iron and bromine.
You must show your working. (4)
(ii) When iron reacts with bromine, bromide ions are formed.
Explain the type of reaction bromine atoms undergo when they are converted to bromide ions. (2)
(Total for Question 5 = 13 marks)
Predict the appearance of astatine. (1)
*(b) The order of reactivity of chlorine, bromine and iodine can be determined by carrying out displacement reactions.
Explain how displacement reactions can be used to show the reactivity of these three elements. (6)
(c) When iron wool is heated in bromine vapour, it reacts to form iron bromide.
(i) In an experiment, 5.60g of iron reacted exactly with 24.0g of bromine, Br2.
[relative atomic masses: Fe = 56.0, Br = 80.0]
Determine, using this information, the balanced equation for the reaction between iron and bromine.
You must show your working. (4)
(ii) When iron reacts with bromine, bromide ions are formed.
Explain the type of reaction bromine atoms undergo when they are converted to bromide ions. (2)
(Total for Question 5 = 13 marks)