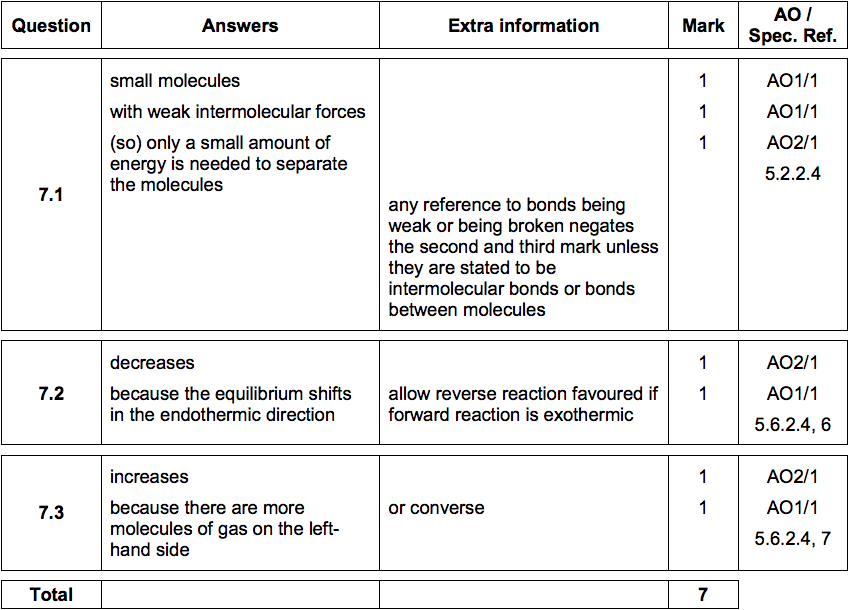
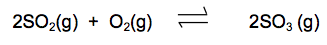q1 Answers
Questions
7) Sulfur dioxide (SO2) is used to manufacture sulfuric acid.
7.1) Explain why sulfur dioxide has a low boiling point. [3 marks]
The equation shows one stage in the manufacture of sulfuric acid from sulfur dioxide.
7.1) Explain why sulfur dioxide has a low boiling point. [3 marks]
The equation shows one stage in the manufacture of sulfuric acid from sulfur dioxide.
The reaction is exothermic in the forward direction.
7.2) Use Le Chatelier’s Principle to predict the effect of increasing the temperature on the amount of sulfur trioxide (SO3) produced at equilibrium.
Give a reason for your answer. [2 marks]
7.3) Use Le Chatelier’s Principle to predict the effect of increasing the pressure on the amount of sulfur trioxide (SO3) produced at equilibrium.
Give a reason for your answer. [2 marks]
7.2) Use Le Chatelier’s Principle to predict the effect of increasing the temperature on the amount of sulfur trioxide (SO3) produced at equilibrium.
Give a reason for your answer. [2 marks]
7.3) Use Le Chatelier’s Principle to predict the effect of increasing the pressure on the amount of sulfur trioxide (SO3) produced at equilibrium.
Give a reason for your answer. [2 marks]
(Total for Question 7 = 7 marks)