Q1
6) Exothermic reactions transfer energy to the surroundings.
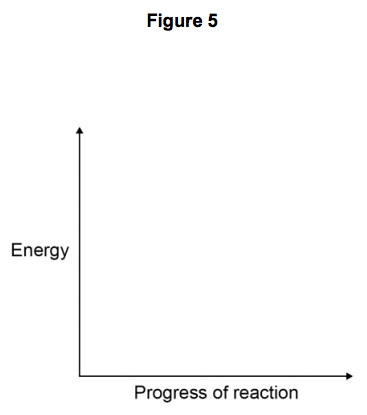
6.1) Draw a reaction profile for an exothermic reaction using the axes in Figure 5.
Show the:
6.1) Draw a reaction profile for an exothermic reaction using the axes in Figure 5.
Show the:
- relative energies of the reactants and products
- activation energy and overall energy change.
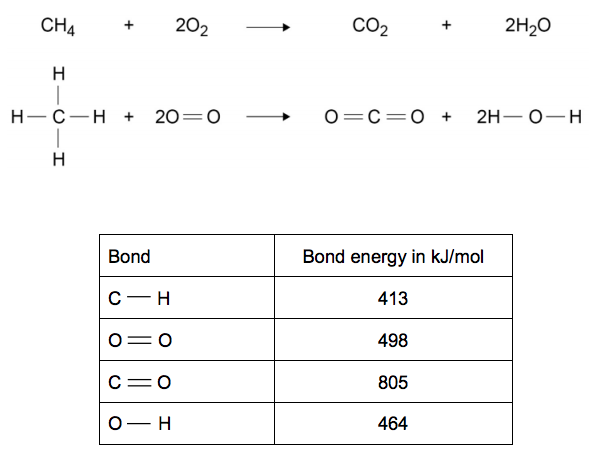
6.2) Combustion is an exothermic reaction.
Calculate the overall energy change for the complete combustion of one mole of methane in oxygen. [3 marks]
Calculate the overall energy change for the complete combustion of one mole of methane in oxygen. [3 marks]
Overall energy change = _____________________ kJ/mol
Figure 6 shows the chemicals given to a student
6.3) The student wants to investigate the reactivity of the four metals.
Outline a plan the student could use to investigate the relative reactivity of the four metals, W, X, Y and Z.
The plan should use the fact that all four metals react exothermically with dilute sulfuric acid.
You should name the apparatus used and comment on the safe use of the chemicals. [6 marks]
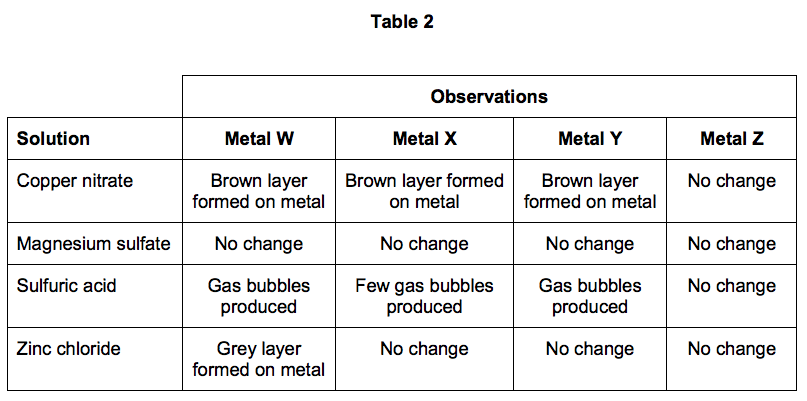
Another student used displacement reactions to investigate the relative reactivity of the four metals, W, X, Y and Z.
Table 2 shows the student’s results.
Outline a plan the student could use to investigate the relative reactivity of the four metals, W, X, Y and Z.
The plan should use the fact that all four metals react exothermically with dilute sulfuric acid.
You should name the apparatus used and comment on the safe use of the chemicals. [6 marks]
Another student used displacement reactions to investigate the relative reactivity of the four metals, W, X, Y and Z.
Table 2 shows the student’s results.
6.4) Give the order of reactivity of metals, W, X, Y and Z.
Use the results in Table 2 to justify your answer. [3 marks]
Use the results in Table 2 to justify your answer. [3 marks]
6.5) The student concluded that these results could also be used to justify the order of reactivity of copper, magnesium, hydrogen and zinc.
The student is not completely correct. Use the results in Table 2 to explain why.
Suggest one further experiment that would provide evidence for the student’s conclusion. [4 marks]
(Total for Question 6 = 18 marks)
The student is not completely correct. Use the results in Table 2 to explain why.
Suggest one further experiment that would provide evidence for the student’s conclusion. [4 marks]
(Total for Question 6 = 18 marks)