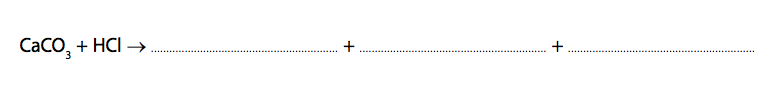Q1 - Paper 2 SPECIMEN (SET 1) Q4 Answers
Questions
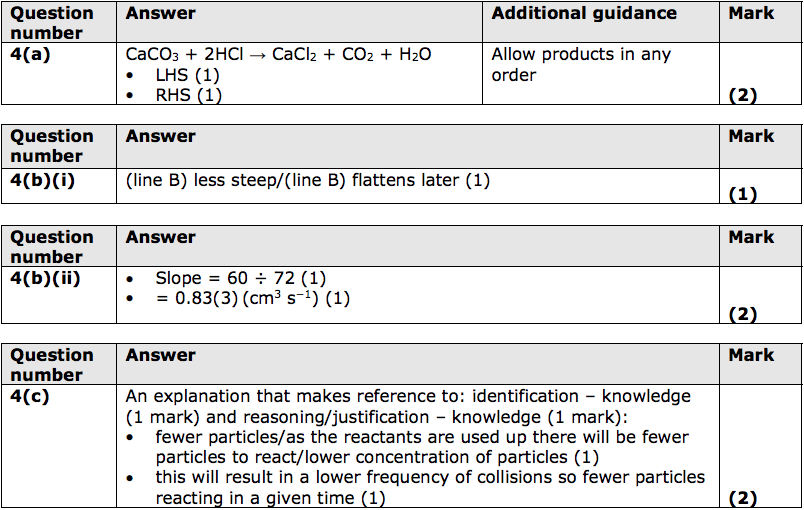
4) A student investigated the rate of reaction between dilute hydrochloric acid and marble chips (calcium carbonate).
Calcium chloride, carbon dioxide and water are formed.
(a) Complete and balance the equation for the reaction. (2)
4) A student investigated the rate of reaction between dilute hydrochloric acid and marble chips (calcium carbonate).
Calcium chloride, carbon dioxide and water are formed.
(a) Complete and balance the equation for the reaction. (2)
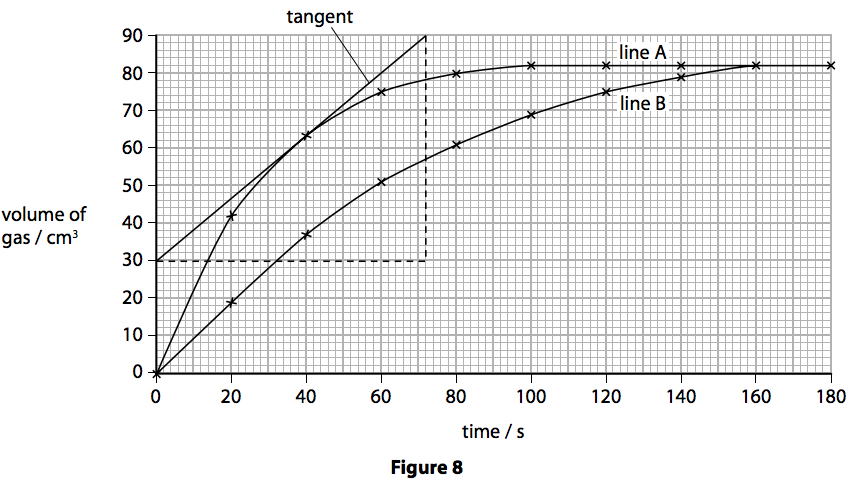
(b) The student investigated the rate by using different sizes of marble chips. In their investigation, the same mass of marble chips was used in each experiment.
The volume of gas given off was measured.
The graph in Figure 8 shows the results.
The volume of gas given off was measured.
The graph in Figure 8 shows the results.
(i) State how the graph shows that line B gives the results for the larger marble chips. (1)
(ii) A tangent has been drawn on line A.
Calculate the rate of reaction at this point. (2)
(ii) A tangent has been drawn on line A.
Calculate the rate of reaction at this point. (2)
rate of reaction =.............................................................cm3s−1
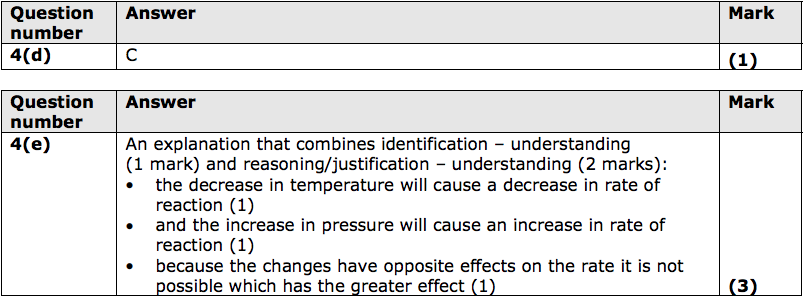
(c) During any reaction, reactants are used up and the rate of reaction decreases.
Explain, in terms of particles, why the rate of reaction decreases. (2)
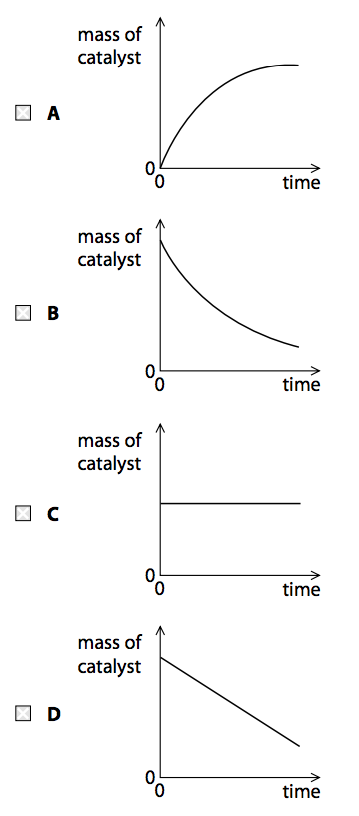
(d) The decomposition of hydrogen peroxide is catalysed by adding a small amount of manganese(IV) oxide. Which of these graphs shows the mass of the catalyst as the reaction takes place? (1)
Explain, in terms of particles, why the rate of reaction decreases. (2)
(d) The decomposition of hydrogen peroxide is catalysed by adding a small amount of manganese(IV) oxide. Which of these graphs shows the mass of the catalyst as the reaction takes place? (1)
(e) Two gases, X and Y, react to give a gaseous product Z.
The reaction is carried out under two different sets of conditions in experiments 1 and 2 as shown in Figure 9.
The reaction is carried out under two different sets of conditions in experiments 1 and 2 as shown in Figure 9.
Explain why it is not possible to predict what the rate of Experiment 2 will be compared with Experiment 1. (3)
(Total for Question 4 = 11 marks)
(Total for Question 4 = 11 marks)