q2 Answers
Questions
(6) The espresso machine shown in Figure 27 is an electrical appliance.
(6) The espresso machine shown in Figure 27 is an electrical appliance.
(a) The espresso machine has an electrical heater connected to a 440V mains supply.
The power of the electrical heater is 3.5kW.
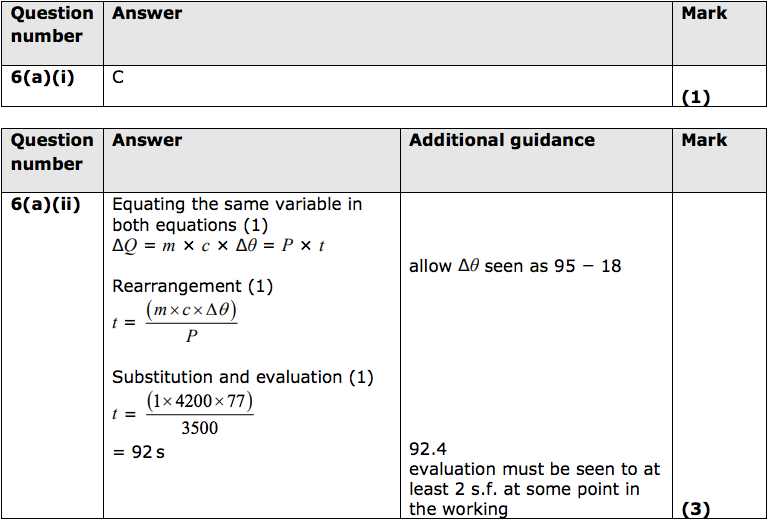
(i) The rating of a fuse is the current above which it melts.
Which of these is the most suitable fuse for the espresso machine circuit? (1)
The power of the electrical heater is 3.5kW.
(i) The rating of a fuse is the current above which it melts.
Which of these is the most suitable fuse for the espresso machine circuit? (1)
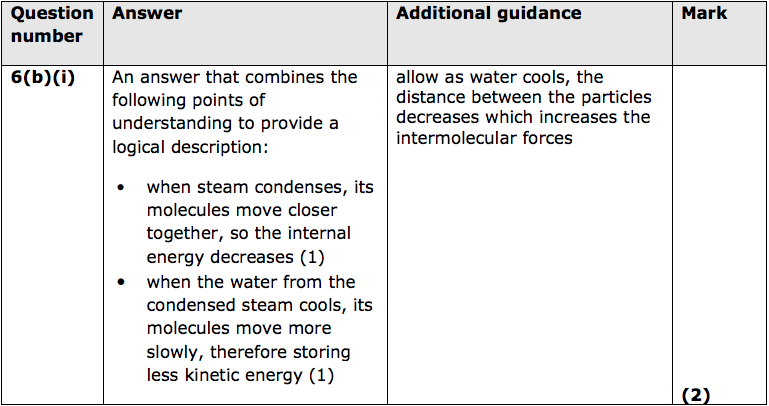
(ii) Before the espresso machine can be used, its heater must raise the temperature of some cold water.
The specific heat capacity of water is 4200J/kg K.
Show that it takes the heater about 90s to raise the temperature of 1kg of water from 18°C to 95°C.
Use an equation from the formula sheet. (3)
(b) The espresso machine has a steam pipe that can be used to heat milk in a jug, as shown in Figure 28.
The specific heat capacity of water is 4200J/kg K.
Show that it takes the heater about 90s to raise the temperature of 1kg of water from 18°C to 95°C.
Use an equation from the formula sheet. (3)
(b) The espresso machine has a steam pipe that can be used to heat milk in a jug, as shown in Figure 28.
Steam from the pipe enters the milk, where steam condenses to water.
The steam and hot water heat the milk.
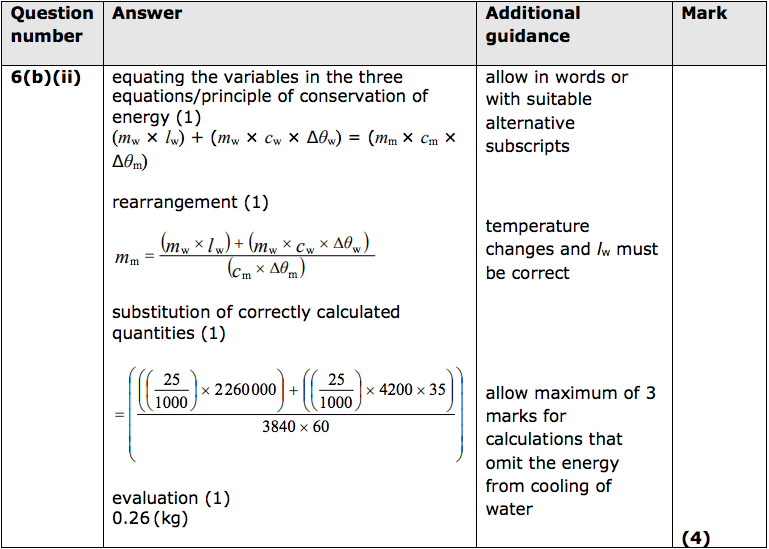
(i) Describe, in terms of energy, how the arrangement and movement of particles in the steam changes as the steam enters the milk, condenses and cools. (2)
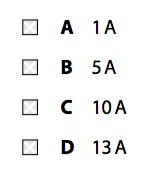
(ii) The specific heat capacity of milk is 3840J/kg K.
The specific heat capacity of water is 4200J/kg K.
The specific latent heat of condensation of steam is 2260kJ/kg.
The temperature of the steam is 100°C.
The mass of steam that condenses is 25g.
The temperature of the milk rises from 5°C to 65°C.
By considering the transfer of energy from the steam to the milk, calculate the mass of milk that is heated by the steam and hot water.
Use equations from the formula sheet. (4)
The steam and hot water heat the milk.
(i) Describe, in terms of energy, how the arrangement and movement of particles in the steam changes as the steam enters the milk, condenses and cools. (2)
(ii) The specific heat capacity of milk is 3840J/kg K.
The specific heat capacity of water is 4200J/kg K.
The specific latent heat of condensation of steam is 2260kJ/kg.
The temperature of the steam is 100°C.
The mass of steam that condenses is 25g.
The temperature of the milk rises from 5°C to 65°C.
By considering the transfer of energy from the steam to the milk, calculate the mass of milk that is heated by the steam and hot water.
Use equations from the formula sheet. (4)
mass of milk = ............................................... kg
(iii) Give two reasons why the actual mass of steam needed to heat the milk from 5°C to 65°C is greater than 25g. (2)
1 ............................................................................................................................................................
2 ............................................................................................................................................................
(Total for Question 6 = 12 marks)
1 ............................................................................................................................................................
2 ............................................................................................................................................................
(Total for Question 6 = 12 marks)