Paper 1 H - SAMPLE SET 1 Q18 Answers
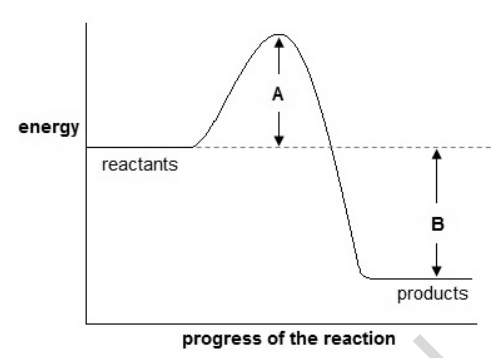
18) Look at the energy profile for a reaction.
(a) What can you deduce about this reaction?
Include the quantities A and B and a full explanation. [4]
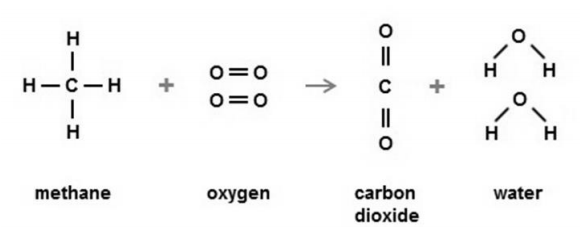
(b) Look at the equation.
Include the quantities A and B and a full explanation. [4]
(b) Look at the equation.
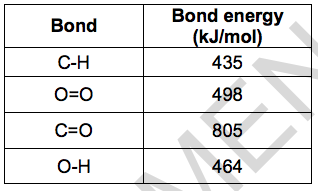
The table shows the bond energies of the bonds involved.
(i) What type of energy change happens when bonds are broken and when bonds are made? [2]
Bonds broken ………………………………
Bonds made ………………………………..
(ii) Calculate the energy change for this reaction. [3]
Bonds broken ………………………………
Bonds made ………………………………..
(ii) Calculate the energy change for this reaction. [3]
Energy change = ………………………. kJ/mol
(c) When propane reacts with oxygen, energy is given out.
Propane gives out 50 kJ/g.
A propane burner is used to boil 200 g of water to make a cup of tea.
The initial temperature of the water is 15°C.
How many grams of propane are needed to heat this water?
Use the following equation:
Energy transferred in J = 4.2 J/g°C x mass of water in g x temperature change in °C
[5]
Propane gives out 50 kJ/g.
A propane burner is used to boil 200 g of water to make a cup of tea.
The initial temperature of the water is 15°C.
How many grams of propane are needed to heat this water?
Use the following equation:
Energy transferred in J = 4.2 J/g°C x mass of water in g x temperature change in °C
[5]
Amount of propane = …………………………. g
(Total for Question 18 = 14 marks)