Paper 1 H - SAMPLE SET 1 Q19 Answers
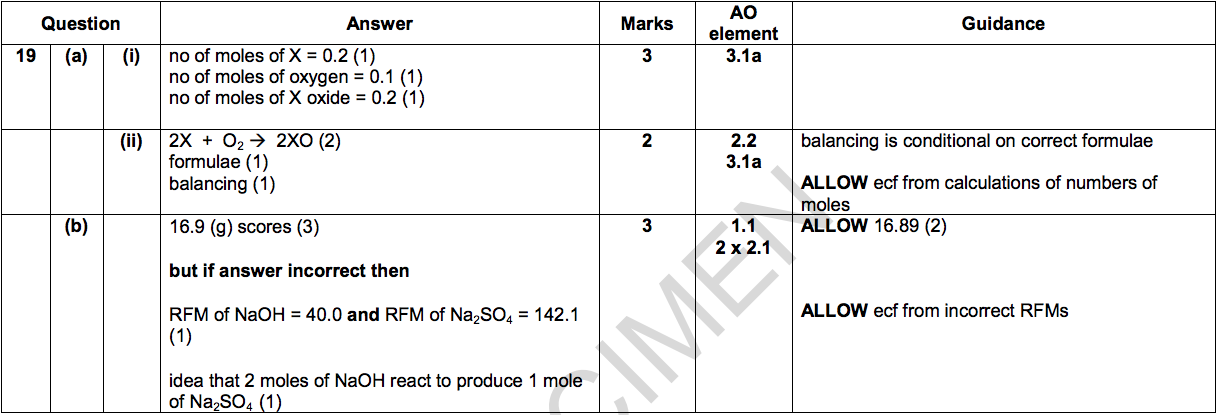
19) Irenka reacts an element, X, with oxygen, O2.
There is one product. It is the oxide of X i.e. X oxide.
4.86 g of X reacts with 3.20 g of oxygen to make 8.06 g of X oxide.
(a) (i) Calculate the number of moles of X, oxygen and X oxide involved in the reaction. [3]
(The relative atomic mass of X is 24.3 and the relative formula mass of oxygen, O2, is 32.0 and of X oxide is 40.3.)
Number of moles of X = ………………………..
Number of moles of O2 = ………………………
Number of moles of X oxide = ………………...
(ii) Use your answers to write the balanced symbol equation for the reaction between X and oxygen to make X oxide. [2]
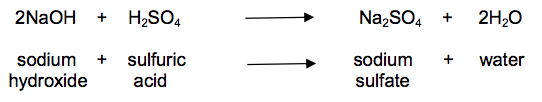
(b) Look at the equation.
It shows the reaction between sodium hydroxide and dilute sulfuric acid.
There is one product. It is the oxide of X i.e. X oxide.
4.86 g of X reacts with 3.20 g of oxygen to make 8.06 g of X oxide.
(a) (i) Calculate the number of moles of X, oxygen and X oxide involved in the reaction. [3]
(The relative atomic mass of X is 24.3 and the relative formula mass of oxygen, O2, is 32.0 and of X oxide is 40.3.)
Number of moles of X = ………………………..
Number of moles of O2 = ………………………
Number of moles of X oxide = ………………...
(ii) Use your answers to write the balanced symbol equation for the reaction between X and oxygen to make X oxide. [2]
(b) Look at the equation.
It shows the reaction between sodium hydroxide and dilute sulfuric acid.
Calculate the mass of sodium hydroxide needed to make 30.0 g of sodium sulfate.
Give your answer to three significant figures. [3]
Give your answer to three significant figures. [3]
Mass of sodium hydroxide = ………………………….. g
(Total for Question 19 =8 marks)