Paper 1 H - SAMPLE SET 1 Q21 Answers
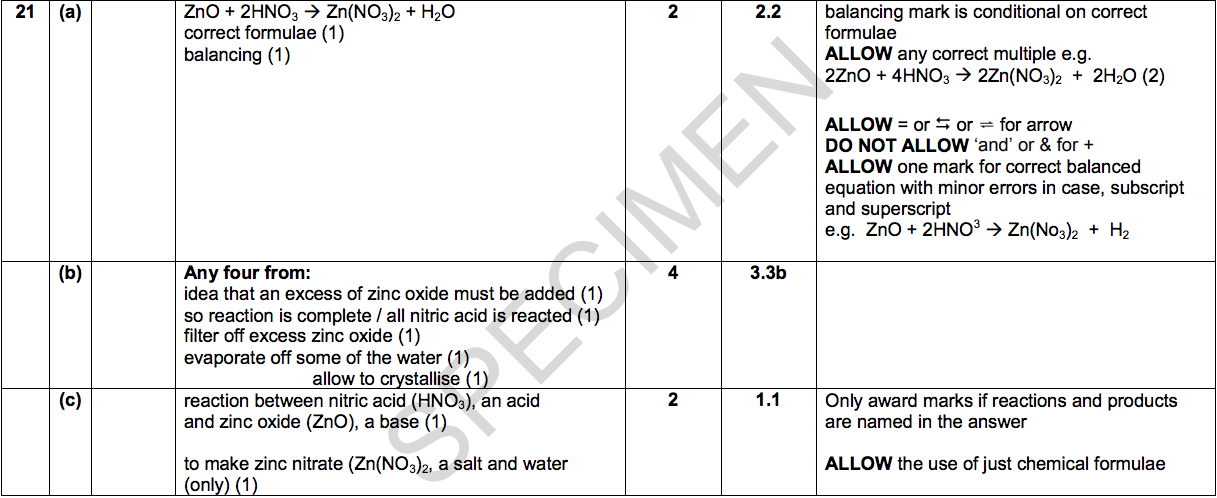
21) Zinc nitrate can be made by reacting zinc oxide with nitric acid, HNO3.
(a) Write a balanced symbol equation for this reaction. [2]
(b) Paul suggests this method for preparing zinc nitrate.
1. Measure 50cm3 of dilute nitric acid into a beaker.
2. Add one spatulaful of zinc oxide.
3. Heat the mixture until crystals of zinc nitrate are made.
Paul’s method will not make a pure dry sample of zinc nitrate.
What improvements should Paul make to the method to make sure that:
> the reaction is complete > the zinc nitrate can be separated from the nitric acid and the zinc oxide?
Explain your answer. [4]
(c) Describe why this reaction is a neutralisation reaction. [2]
(Total for Question 21 = 8 marks)
(a) Write a balanced symbol equation for this reaction. [2]
(b) Paul suggests this method for preparing zinc nitrate.
1. Measure 50cm3 of dilute nitric acid into a beaker.
2. Add one spatulaful of zinc oxide.
3. Heat the mixture until crystals of zinc nitrate are made.
Paul’s method will not make a pure dry sample of zinc nitrate.
What improvements should Paul make to the method to make sure that:
> the reaction is complete > the zinc nitrate can be separated from the nitric acid and the zinc oxide?
Explain your answer. [4]
(c) Describe why this reaction is a neutralisation reaction. [2]
(Total for Question 21 = 8 marks)