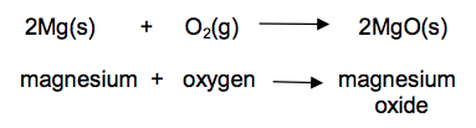Paper 1 H - SAMPLE SET 1 Q22 Answers
22) Magnesium burns in oxygen to make magnesium oxide.
The reaction involves both oxidation and reduction.
The reaction involves both oxidation and reduction.
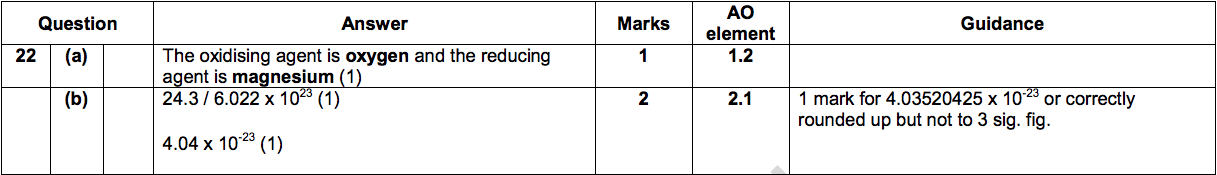
(a) Complete the sentence. [1]
During this reaction, the oxidising agent is ……………………… ......... and the reducing agent is ……………………................ .
(b) Magnesium has an atomic number of 12.
Calculate the mean mass of an atom of magnesium. Quote your answer to three significant figures. [2]
(Avogadro constant = 6.022 x 1023 atoms per mole)
During this reaction, the oxidising agent is ……………………… ......... and the reducing agent is ……………………................ .
(b) Magnesium has an atomic number of 12.
Calculate the mean mass of an atom of magnesium. Quote your answer to three significant figures. [2]
(Avogadro constant = 6.022 x 1023 atoms per mole)
Mean mass ……………………… g
(Total for Question 22 = 3 marks)