Paper 1 H - SAMPLE SET 1 Q23 Answers
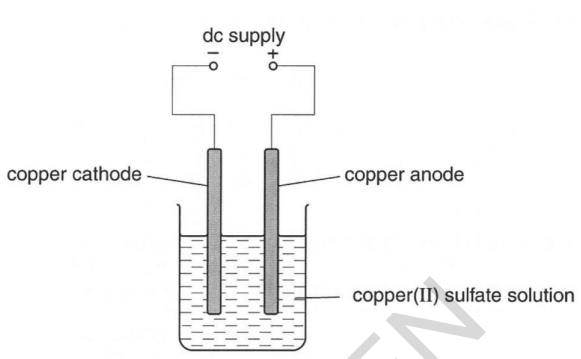
23) Meena electrolyses copper sulfate using copper electrodes.
Look at the diagram. It shows the apparatus she uses.
Look at the diagram. It shows the apparatus she uses.
She investigates the change in mass at each electrode before and after the electrolysis.
Look at Meena’s method.
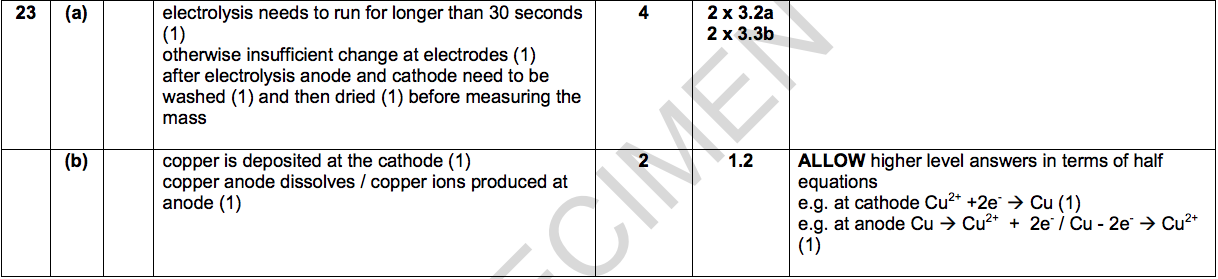
(a) What improvements could you make to Meena’s experiment?
Explain your answers. [4]
(b) Meena finds that
Explain these observations in terms of the reactions at each electrode. [2]
(Total for Question 23 = 6 marks)
Look at Meena’s method.
- Using a balance, measure the mass of the copper cathode and copper anode.
- Set up the apparatus and run the electrolysis for 30 seconds.
- Remove the copper cathode and the copper anode and immediately place them on the balance and measure their masses again.
(a) What improvements could you make to Meena’s experiment?
Explain your answers. [4]
(b) Meena finds that
- the cathode gains mass
- the anode loses mass.
Explain these observations in terms of the reactions at each electrode. [2]
(Total for Question 23 = 6 marks)