Paper 1 H - SAMPLE SET 1 Q24
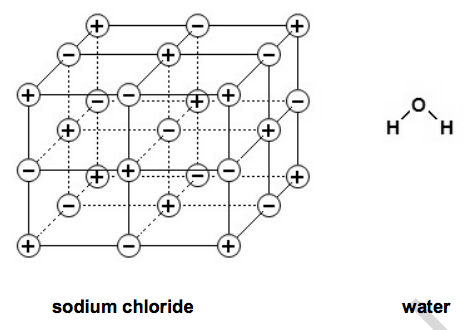
24) Look at the diagrams.
They show the structures of two compounds.
They show the structures of two compounds.
(a) Sodium chloride has a melting point of 801oC. Use the structure of sodium chloride to explain why. [2]
(b) Water has a low melting point and boiling point. Explain why. [2]
(c) Magnesium oxide has a similar structure to sodium chloride.
Draw ‘dot and cross’ diagrams to show the ionic bonding in magnesium oxide.
You should include the charges on the ions.
The electronic structure of magnesium is 2.8.2.
The electronic structure of oxygen is 2.6.
[3]
(Total for Question 24 = 7 marks)
(b) Water has a low melting point and boiling point. Explain why. [2]
(c) Magnesium oxide has a similar structure to sodium chloride.
Draw ‘dot and cross’ diagrams to show the ionic bonding in magnesium oxide.
You should include the charges on the ions.
The electronic structure of magnesium is 2.8.2.
The electronic structure of oxygen is 2.6.
[3]
(Total for Question 24 = 7 marks)