Paper 2 H - SAMPLE SET 1 Q17
17) The Group 7 elements are known as the halogens.
The halogens have similar chemical properties.
Their physical properties vary with increasing atomic number.
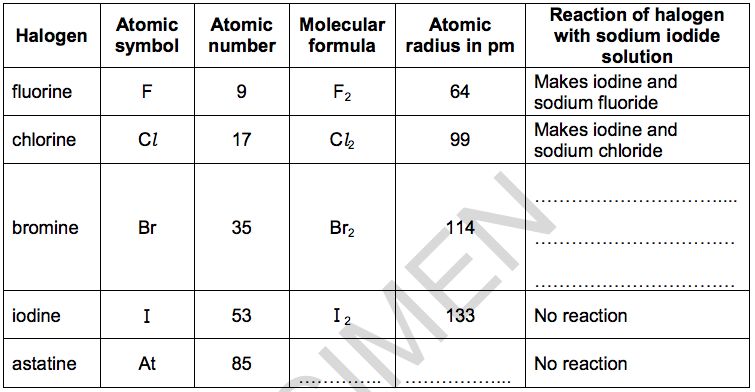
(a) Look at the table of information about the halogens.
The halogens have similar chemical properties.
Their physical properties vary with increasing atomic number.
(a) Look at the table of information about the halogens.
(i) Predict the molecular formula and atomic radius of astatine.
Put your answers in the table. [2]
(ii) Predict the reaction of bromine with sodium iodide solution.
Put your answer in the table. [1]
(iii) Explain your answer to (ii) in terms of the reactivity of the halogens. [1]
(b) All halogens react with alkali metals to make a salt.
(i) All halogens have similar chemical reactions.
Explain why in terms of electronic structure. [1]
(ii) Sodium reacts with bromine to make sodium bromide, NaBr.
Construct the balanced symbol equation for this reaction. [2]
(iii) What is the formula of the product of the reaction between astatine and potassium? [1]
(Total for Question 17 = 8 marks)
Put your answers in the table. [2]
(ii) Predict the reaction of bromine with sodium iodide solution.
Put your answer in the table. [1]
(iii) Explain your answer to (ii) in terms of the reactivity of the halogens. [1]
(b) All halogens react with alkali metals to make a salt.
(i) All halogens have similar chemical reactions.
Explain why in terms of electronic structure. [1]
(ii) Sodium reacts with bromine to make sodium bromide, NaBr.
Construct the balanced symbol equation for this reaction. [2]
(iii) What is the formula of the product of the reaction between astatine and potassium? [1]
(Total for Question 17 = 8 marks)