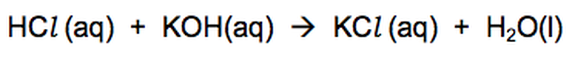Paper 2 H - SAMPLE SET 1 Q19 Answers
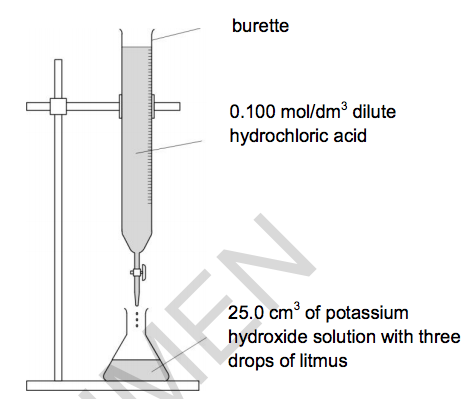
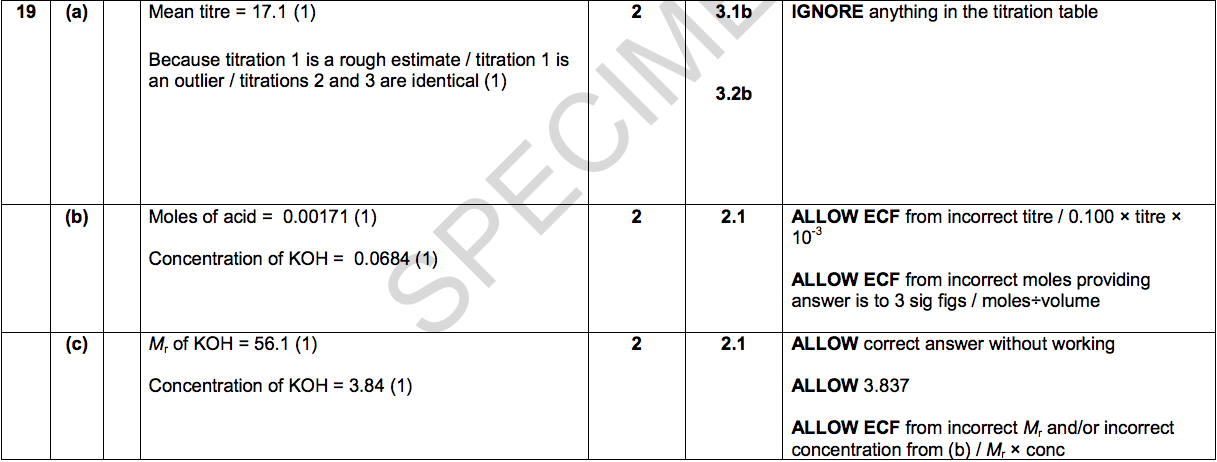
19) Sarah does three titrations with dilute hydrochloric acid and potassium hydroxide solution.
Hydrochloric acid neutralises the alkali potassium hydroxide
Hydrochloric acid neutralises the alkali potassium hydroxide
Look at the apparatus she uses.
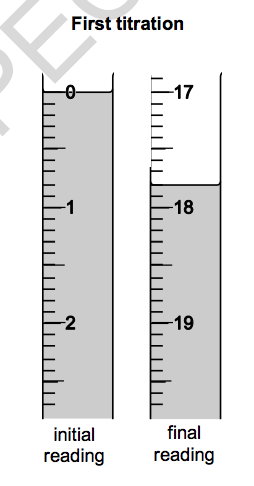
Look at the diagrams. They show parts of the burette during the first titration.
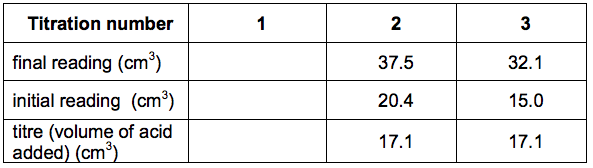
Here is Sarah’s results table:
(a) Use the diagrams and table to help you calculate the mean titre.
Explain your answer. [2]
Explain your answer. [2]
Mean titre = …………….. cm3
(b) Sarah uses 25.0 cm3 of potassium hydroxide solution, KOH.
She also uses hydrochloric acid with a concentration of 0.100 mol/dm3.
Calculate the concentration, in mol/dm3, of the KOH(aq). [2]
Concentration of KOH(aq) = ………………….. mol/dm3
(c) Use your answer to (b) to calculate the concentration of the KOH(aq) in g/dm3.
Concentration of KOH(aq) = ………………….. g/dm3
(Total for Question 19 = 6 marks)