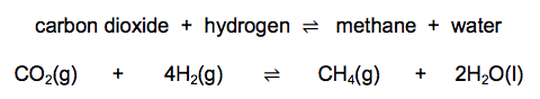Paper 2 H - SAMPLE SET 1 Q21
21) The reversible reaction between carbon dioxide and hydrogen makes methane and water.
(a) In a sealed container this reversible reaction forms a dynamic equilibrium.
What is meant by the term dynamic equilibrium?
Refer to both concentration and rate of reaction in your answer. [2]
(b) Kayvan investigates this reaction.
He predicts that 11.0 g of carbon dioxide should make 4.0 g of methane.
In an experiment, he finds that 11.0 g of carbon dioxide makes 2.2 g of methane.
Calculate the percentage yield of methane. [2]
What is meant by the term dynamic equilibrium?
Refer to both concentration and rate of reaction in your answer. [2]
(b) Kayvan investigates this reaction.
He predicts that 11.0 g of carbon dioxide should make 4.0 g of methane.
In an experiment, he finds that 11.0 g of carbon dioxide makes 2.2 g of methane.
Calculate the percentage yield of methane. [2]
Percentage yield = ……………………%
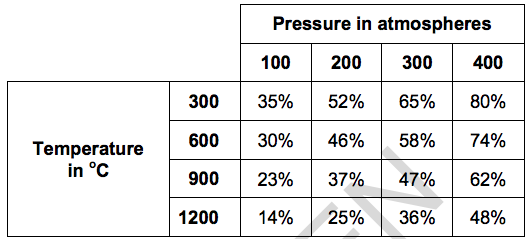
(c)* Kayvan investigates the effect of changing the pressure and changing the temperature on this reaction.
The table shows the percentage yield of methane in the equilibrium mixture under different conditions.
Kayvan predicts that the reaction between carbon dioxide and hydrogen is endothermic and involves a reduction in the volume of gases.
Describe and explain whether Kayvan’s predictions are supported by the reaction and results in the table. [6]
(Total for Question 21 = 10 marks)
Describe and explain whether Kayvan’s predictions are supported by the reaction and results in the table. [6]
(Total for Question 21 = 10 marks)