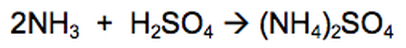Paper 2 H - SAMPLE SET 1 Q22 Answers
22) Ammonium sulfate, (NH4)2SO4, is a fertiliser.
Ammonium sulfate can be manufactured from ammonia and sulfuric acid.
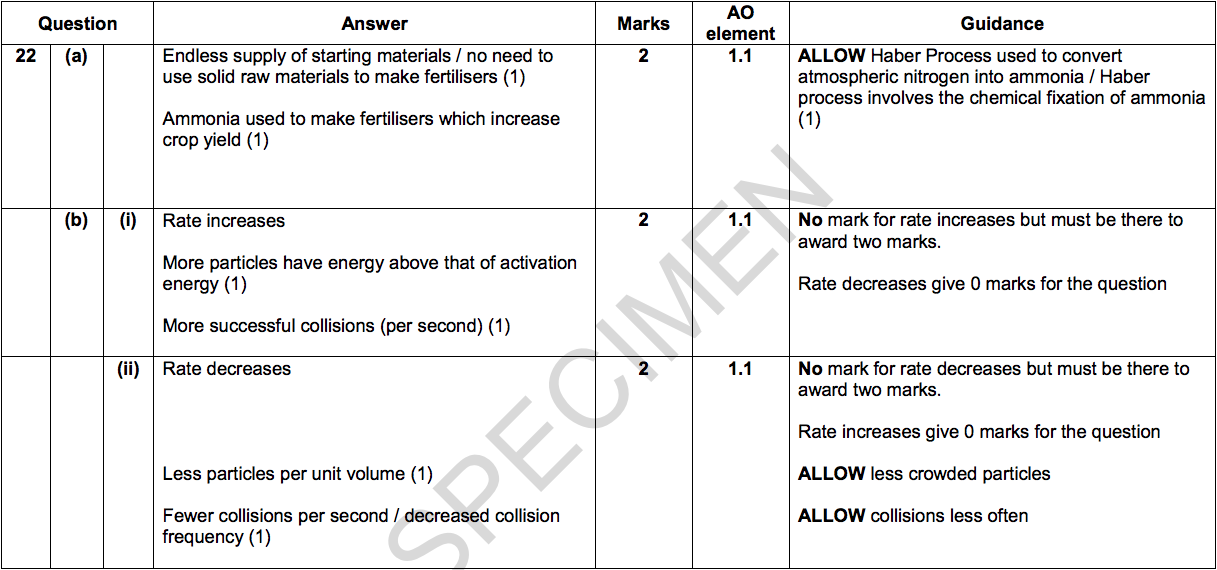
(a) The Haber Process is used to manufacture ammonia. Scientists think that the Haber Process is one of the most important chemical reactions.
Explain the importance of the Haber Process in agriculture. [2]
(b) The Contact Process is used to manufacture sulfuric acid.
The Contact Process involves the reaction between sulfur dioxide and oxygen.
The conditions used are 450°C and about 10 atmospheres pressure.
(i) If the temperature is increased to 500°C the rate of reaction changes.
Describe and explain this change in rate of reaction. [2]
(ii) If the pressure is reduced to 5 atmospheres the rate of reaction changes.
Describe and explain this change in rate of reaction. [2]
(c) Ammonium sulfate is a salt.
It is made using the reaction between the alkali ammonia and sulfuric acid.
Ammonium sulfate can be manufactured from ammonia and sulfuric acid.
(a) The Haber Process is used to manufacture ammonia. Scientists think that the Haber Process is one of the most important chemical reactions.
Explain the importance of the Haber Process in agriculture. [2]
(b) The Contact Process is used to manufacture sulfuric acid.
The Contact Process involves the reaction between sulfur dioxide and oxygen.
The conditions used are 450°C and about 10 atmospheres pressure.
(i) If the temperature is increased to 500°C the rate of reaction changes.
Describe and explain this change in rate of reaction. [2]
(ii) If the pressure is reduced to 5 atmospheres the rate of reaction changes.
Describe and explain this change in rate of reaction. [2]
(c) Ammonium sulfate is a salt.
It is made using the reaction between the alkali ammonia and sulfuric acid.
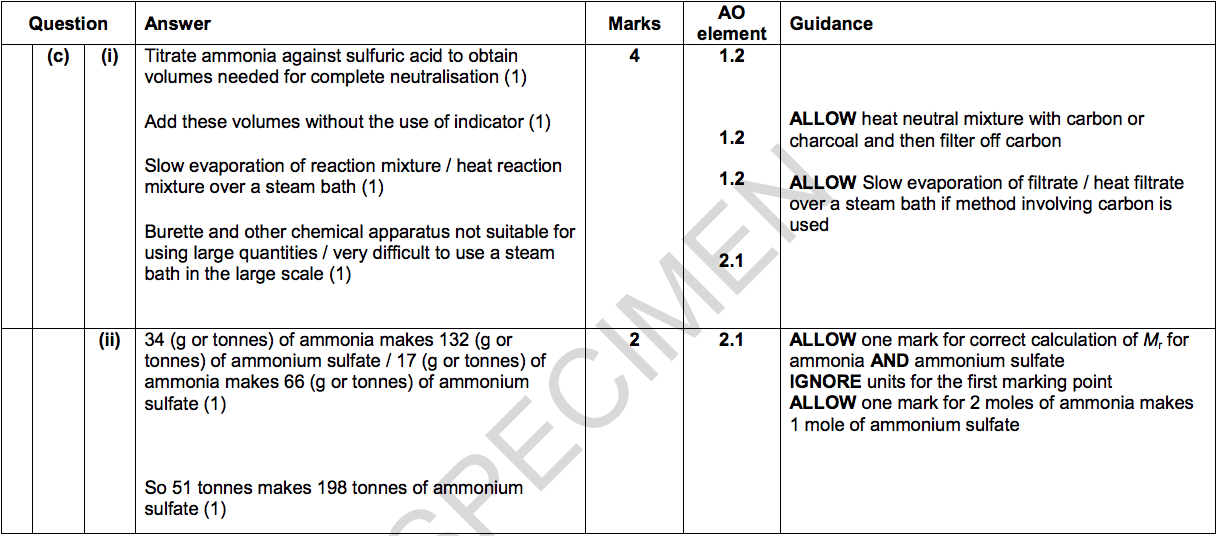
(i) Describe how a sample of solid ammonium sulfate is prepared in a laboratory.
Explain why this method is not suitable to be used industrially. [4]
(ii) Predict the maximum mass of ammonium sulfate that can be made from 51 tonnes of ammonia. [2]
Explain why this method is not suitable to be used industrially. [4]
(ii) Predict the maximum mass of ammonium sulfate that can be made from 51 tonnes of ammonia. [2]
Maximum mass = ……………….. tonnes
(Total for Question 22 = 12 marks)