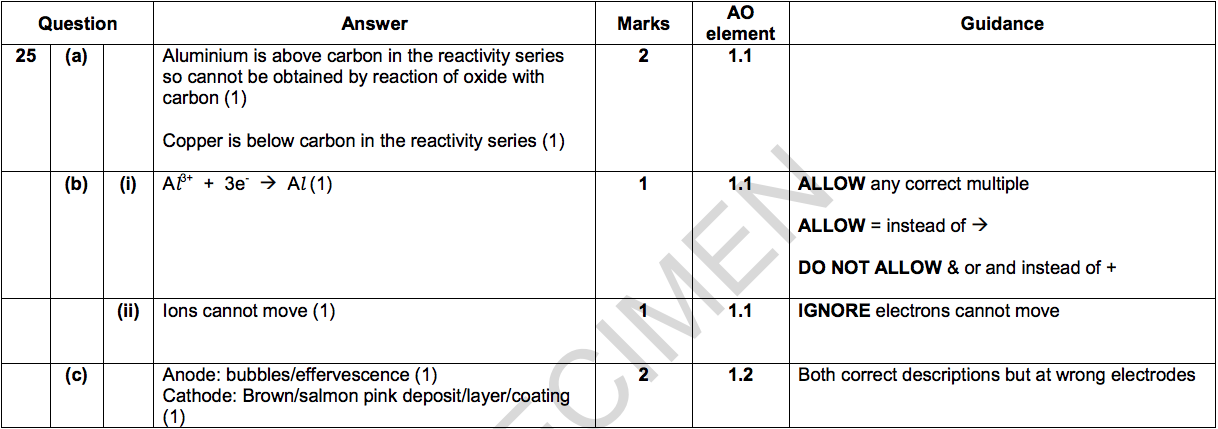
Paper 2 H - SAMPLE SET 1 Q25 Answers
25) Aluminium is extracted from its ore using electrolysis.
Copper is extracted from its ore by heating with carbon.
(a) Explain why different methods are used to extract aluminium and copper. [2]
(b) Molten aluminium oxide contains Al3+ and O2- ions.
The electrolysis of molten aluminium oxide makes aluminium and oxygen.
(i) Write the balanced symbol equation for the electrode reaction that happens at the cathode.
Use the symbol e- to represent an electron. [1]
(ii) Solid aluminium oxide cannot be electrolysed.
Explain why. [1]
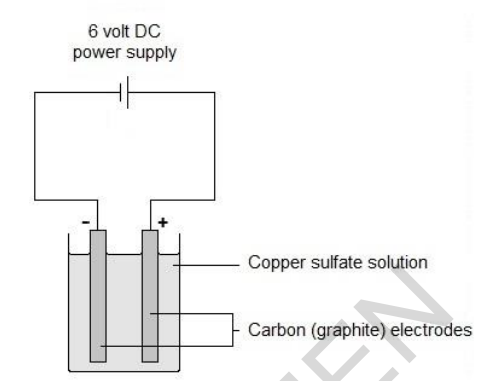
(c) Copper is also made by electrolysis of copper sulfate solution.
Look at the diagram of the apparatus used in this electrolysis.
Copper is extracted from its ore by heating with carbon.
(a) Explain why different methods are used to extract aluminium and copper. [2]
(b) Molten aluminium oxide contains Al3+ and O2- ions.
The electrolysis of molten aluminium oxide makes aluminium and oxygen.
(i) Write the balanced symbol equation for the electrode reaction that happens at the cathode.
Use the symbol e- to represent an electron. [1]
(ii) Solid aluminium oxide cannot be electrolysed.
Explain why. [1]
(c) Copper is also made by electrolysis of copper sulfate solution.
Look at the diagram of the apparatus used in this electrolysis.
Describe what you would see at each of the electrodes. [2]
At the anode: ................................................................
At the cathode: .............................................................
(Total for Question 25 = 6 marks)
At the anode: ................................................................
At the cathode: .............................................................
(Total for Question 25 = 6 marks)