1.5 Isotopes & Ions
|
Video coming soon!
|
Atoms are tiny particles that make up every item in the universe. Atoms are made up of three sub atomic particles:
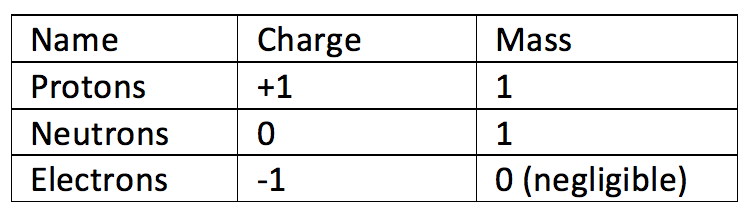
- Protons – a positively charged sub atomic particle (+1)
- Neutrons – a neutrally charged sub atomic particle (0)
- Electrons – a negatively charged subatomic particle (-1)
Protons and neutrons are found in the centre of an atom, which is known as the nucleus. The nucleus is tiny in comparison to the size of the rest of the atom but it makes up most of the mass of the atom. Protons and neutrons have a mass of 1. Electrons move around the nucleus in shells. The first electronic shell holds 2 electrons, the second and third hold 8. Electrons will fill the electron shell closest to the nucleus first, and then fill the shells that are further out.
It is the number of protons that determines what element an atom is. Atoms should have no overall electrical charge, which means that the number of protons will be equal to the number of electrons. Protons and electrons have the same magnitude of charge but in opposite directions; protons have a positive charge and electrons have a negative charge. Neutrons have no charge and therefore they have no effect on the charge of the atom.
It is the number of protons that determines what element an atom is. Atoms should have no overall electrical charge, which means that the number of protons will be equal to the number of electrons. Protons and electrons have the same magnitude of charge but in opposite directions; protons have a positive charge and electrons have a negative charge. Neutrons have no charge and therefore they have no effect on the charge of the atom.
Ions
Atoms can lose or gain electrons. This results in the atom becoming positively or negatively charged and we called atoms that have a charge (either positive or negative charge) an ion.
Let’s assume that we have an element. We assume that elements are neutrally charged. This means that the number of electrons in the element is equal to the number of protons in the element. The charges of the protons and neutrons will balance out, thus meaning that the element has a no charge. For example, a boron atom will have 5 protons (the atomic number), 5 electrons (because the number of electrons must equal the number of protons in order for the atom to be neutrally charged) and 6 neutrons (the relative atomic mass minus the number of protons: 11-5).
Let’s now assume that our neutrally charged atom gains an electron. This results in the atom having one more electron (6) than protons (5), which causes the atom becoming negatively charged with a magnitude of -1.
Now, rather than boron gaining an electron, it is now going to lose an electron. If boron loses an electron, the number of electrons that boron has is 4. Boron has 5 protons and only 4 electrons which means that it has a positive charge (the charge is +1).
So, if a neutrally charged atom gains an electron it becomes negatively charged, and if it loses an electron is becomes positively charged. Either loosing or gaining an electron results in the atom becoming an ion (if it started off being neutrally charged and we assume that elements are neutrally charged).
We denote the charge of an atom by placing the charge in the superscript. For example, when boron gains an electron it becomes negative charged (one more electron than protons). We write boron as B-. There is no need to place a 1 before the minus sign because if there is no number next to the minus we automatically assume that it is a 1 minus (this is similar to in maths where we write 1x as just x).
Atoms can lose or gain more than one electron. For example, let’s assume that magnesium loses two electrons. This results magnesium having two more protons than electrons, resulting in it having a charge of 2+. We write out magnesium as Mg2+.
Atoms can lose or gain electrons. This results in the atom becoming positively or negatively charged and we called atoms that have a charge (either positive or negative charge) an ion.
Let’s assume that we have an element. We assume that elements are neutrally charged. This means that the number of electrons in the element is equal to the number of protons in the element. The charges of the protons and neutrons will balance out, thus meaning that the element has a no charge. For example, a boron atom will have 5 protons (the atomic number), 5 electrons (because the number of electrons must equal the number of protons in order for the atom to be neutrally charged) and 6 neutrons (the relative atomic mass minus the number of protons: 11-5).
Let’s now assume that our neutrally charged atom gains an electron. This results in the atom having one more electron (6) than protons (5), which causes the atom becoming negatively charged with a magnitude of -1.
Now, rather than boron gaining an electron, it is now going to lose an electron. If boron loses an electron, the number of electrons that boron has is 4. Boron has 5 protons and only 4 electrons which means that it has a positive charge (the charge is +1).
So, if a neutrally charged atom gains an electron it becomes negatively charged, and if it loses an electron is becomes positively charged. Either loosing or gaining an electron results in the atom becoming an ion (if it started off being neutrally charged and we assume that elements are neutrally charged).
We denote the charge of an atom by placing the charge in the superscript. For example, when boron gains an electron it becomes negative charged (one more electron than protons). We write boron as B-. There is no need to place a 1 before the minus sign because if there is no number next to the minus we automatically assume that it is a 1 minus (this is similar to in maths where we write 1x as just x).
Atoms can lose or gain more than one electron. For example, let’s assume that magnesium loses two electrons. This results magnesium having two more protons than electrons, resulting in it having a charge of 2+. We write out magnesium as Mg2+.
Isotopes
Isotopes are atoms of an element with the correct number of protons and electrons, but different numbers of neutrons. As isotopes have the same number of protons, it means that the atoms are the same element. The atoms will have the same atomic number (the atomic number is the number of protons in the atoms). However, because the isotopes have a different number of neutrons, the mass numbers will be different (the mass number is the sum of the number of protons and the number of neutrons).
A common example of isotopes that comes up in the exam is carbon-12 and carbon-14. Both isotopes have 6 protons, hence why they are both carbon. They will both have a neutral charge, meaning that they will both have 6 electrons. However, they will have a different number of neutrons. We work out the number of neutrons by taking the atomic number (number of protons) from the mass number. For carbon-12, we have 6 neutrons (12 - 6 = 6). For carbon-14, we have 8 neutrons (14 – 6 = 8), which is 2 more than carbon-12.
Most elements have many isotopes, but each element only has one or two isotopes that are stable. The other isotopes are unstable and radioactive. This means that they give out radiation and decay into other elements. Radioactive decay is what this section is all about.
Isotopes are atoms of an element with the correct number of protons and electrons, but different numbers of neutrons. As isotopes have the same number of protons, it means that the atoms are the same element. The atoms will have the same atomic number (the atomic number is the number of protons in the atoms). However, because the isotopes have a different number of neutrons, the mass numbers will be different (the mass number is the sum of the number of protons and the number of neutrons).
A common example of isotopes that comes up in the exam is carbon-12 and carbon-14. Both isotopes have 6 protons, hence why they are both carbon. They will both have a neutral charge, meaning that they will both have 6 electrons. However, they will have a different number of neutrons. We work out the number of neutrons by taking the atomic number (number of protons) from the mass number. For carbon-12, we have 6 neutrons (12 - 6 = 6). For carbon-14, we have 8 neutrons (14 – 6 = 8), which is 2 more than carbon-12.
Most elements have many isotopes, but each element only has one or two isotopes that are stable. The other isotopes are unstable and radioactive. This means that they give out radiation and decay into other elements. Radioactive decay is what this section is all about.