Back to C1 Home
C1 H) Evaporation & Crystallisation
C1 H) Evaporation & Crystallisation
We can separate a solid from a liquid (such as water) by using filtration, evaporation or crystallisation. Filtration is used when the solid is insoluble (not able to dissolve), and evaporation or crystallisation is used when the solid is soluble (able to dissolve).
Filtration – Insoluble Solid
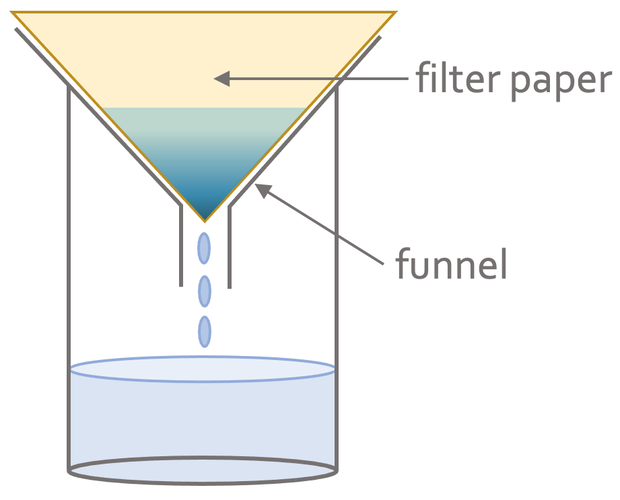
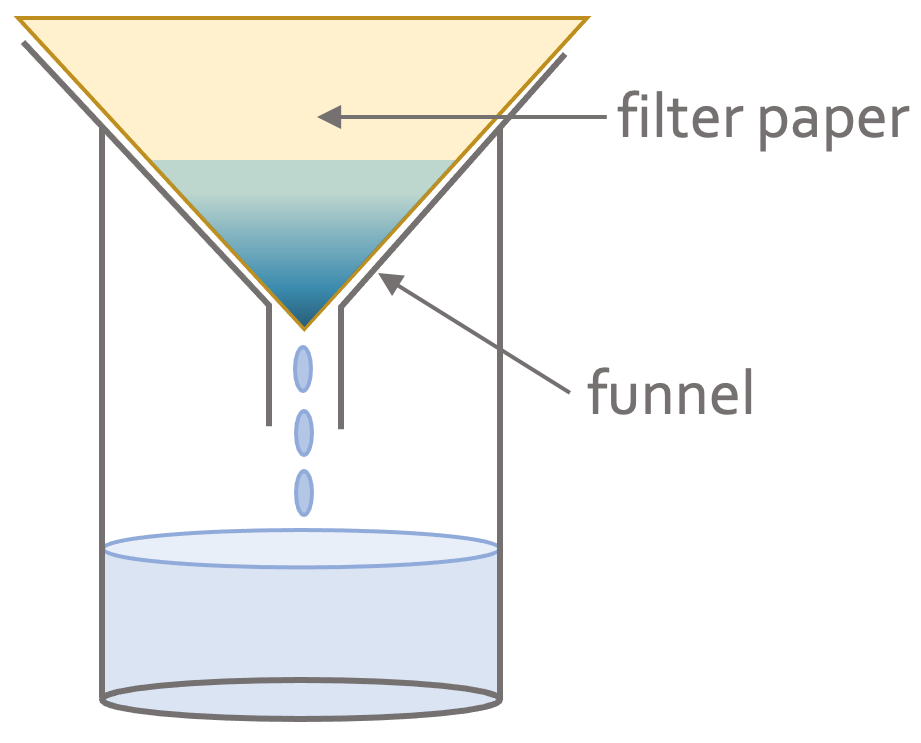
Filtration is used when the solid is insoluble, which means that the solid does not dissolve in a liquid. We can separate the insoluble solid from a liquid by pouring the mixture through a funnel that is lined with filter paper. The filter paper will allow the liquid to pass through and will not allow the insoluble solid through. This means that the insoluble solid will be left on the filter paper. The solid that is left on the filter paper is known as the residue and what passes through the filter paper is known as the filtrate. The apparatus will be set up like the diagram below.
Filtration is used when the solid is insoluble, which means that the solid does not dissolve in a liquid. We can separate the insoluble solid from a liquid by pouring the mixture through a funnel that is lined with filter paper. The filter paper will allow the liquid to pass through and will not allow the insoluble solid through. This means that the insoluble solid will be left on the filter paper. The solid that is left on the filter paper is known as the residue and what passes through the filter paper is known as the filtrate. The apparatus will be set up like the diagram below.
An example would be the filtering of a mixture of water and sand (sand is insoluble in water). The first step in separating the water and sand is to pour the mixture into the funnel that is lined with filter paper. The water will pass through the filter paper into the beaker (the water will be the filtrate). The sand will be unable to pass through the filter paper and will be left on the filter paper as residue.
Soluble Solids
Soluble solids are solids that dissolve in a liquid. We can separate a soluble solid from a solution in two different ways; evaporation and crystallisation. Both of these ways remove the solvent (the liquid) from a solution (which is a liquid with a soluble solid dissolved in the liquid). The methods for evaporation and crystallisation are very similar.
Evaporation
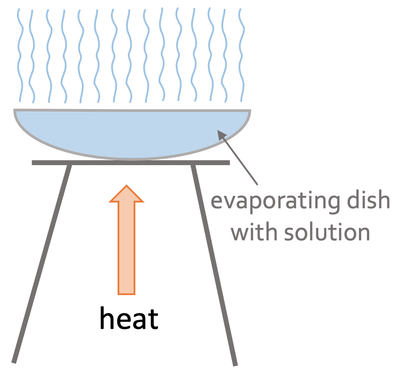
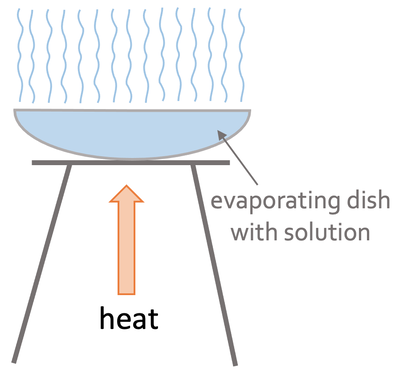
Evaporation is the quicker of the two methods. We set up the apparatus like what is shown below.
Soluble solids are solids that dissolve in a liquid. We can separate a soluble solid from a solution in two different ways; evaporation and crystallisation. Both of these ways remove the solvent (the liquid) from a solution (which is a liquid with a soluble solid dissolved in the liquid). The methods for evaporation and crystallisation are very similar.
Evaporation
Evaporation is the quicker of the two methods. We set up the apparatus like what is shown below.
We pour our mixture into the evaporating dish. We then slowly heat the solution using a Bunsen burner. The heating causes the solvent (liquid) to start evaporating, which results in the solution becoming more concentrated; the solution becomes more concentrated because there is the same amount of the soluble solid and less of the solvent as some of the solvent has evaporated. After heating the solution for a certain period of time, crystals of the soluble solid will start to form. We continue heating the solution until we are left with dry crystals.
Crystallisation
If the soluble solid that we are separating from a solution breaks down when it is heated, we need to use crystallisation instead of evaporation. Crystallisation is slower than evaporation.
The start of crystallisation is exactly the same as evaporation. We pour our solution into an evaporating dish and gently heat the solution using a Bunsen burner. The heating causes the solvent to start evaporating. After some time, we will notice crystals starting to form in the solution; this is known as the point of crystallisation. When this happens, we remove the evaporating dish from the heat and leave the solution somewhere to cool. The soluble solid will start to form larger crystals as the solid becomes insoluble in the cool more concentrated solution (the solution is now more concentrated because some of the solvent has evaporated). We then filter the crystals out of the solution, and leave the crystals in a warm place to dry (we filter the crystals by using the same method that was outlined at the start of this section). We can speed up the drying process by placing the crystals in a drying oven or a desiccator.
An example of crystallisation is the separating of copper sulfate from a copper sulfate solution.
Crystallisation
If the soluble solid that we are separating from a solution breaks down when it is heated, we need to use crystallisation instead of evaporation. Crystallisation is slower than evaporation.
The start of crystallisation is exactly the same as evaporation. We pour our solution into an evaporating dish and gently heat the solution using a Bunsen burner. The heating causes the solvent to start evaporating. After some time, we will notice crystals starting to form in the solution; this is known as the point of crystallisation. When this happens, we remove the evaporating dish from the heat and leave the solution somewhere to cool. The soluble solid will start to form larger crystals as the solid becomes insoluble in the cool more concentrated solution (the solution is now more concentrated because some of the solvent has evaporated). We then filter the crystals out of the solution, and leave the crystals in a warm place to dry (we filter the crystals by using the same method that was outlined at the start of this section). We can speed up the drying process by placing the crystals in a drying oven or a desiccator.
An example of crystallisation is the separating of copper sulfate from a copper sulfate solution.
Separating Rock Salt
Rock salt is a mixture of salt and sand. The salt in rock salt is soluble (will dissolve in water) and the sand is insoluble (won’t dissolve in water). Here are the steps that we use to separate the sand and salt in rock salt.
We grind the rock salt up to make the salt crystals small so that they dissolve well in water (the small salt crystals will have a greater surface area, which means that they will dissolve quicker/ more easily). We then add the grinded rock salt to a beaker of water and stir to help the salt dissolve in the water. We now have a solution with the salt dissolved in the water, and the sand in the water but not dissolved in the water (the sand will not dissolve because sand is insoluble).
The next step is to filter the mixture, which we do by pouring the mixture through a funnel that is lined with filter paper. The water and the dissolved salt will pass through the filter paper, but the insoluble sand will not – the sand will be left on the filter paper (the sand will be the residue). We then collect the sand off of the filter paper.
The solution that has passed through the filter paper (the filtrate) is water with dissolved salt. We can separate the salt from the water by using evaporation. We pour the filtrate into an evaporating dish and heat it up using a Bunsen burner. The heat will cause the water to evaporate, which will leave us with salt crystals. Instead of evaporation, we could have used crystallisation to obtain the salt crystals (the salt in rock salt won’t break down or react when heat is applied, but if the salt did break down or react when heat is applied, we would have had to use crystallisation instead of evaporation).
We now have separated the sand and salt in rock salt.
Rock salt is a mixture of salt and sand. The salt in rock salt is soluble (will dissolve in water) and the sand is insoluble (won’t dissolve in water). Here are the steps that we use to separate the sand and salt in rock salt.
We grind the rock salt up to make the salt crystals small so that they dissolve well in water (the small salt crystals will have a greater surface area, which means that they will dissolve quicker/ more easily). We then add the grinded rock salt to a beaker of water and stir to help the salt dissolve in the water. We now have a solution with the salt dissolved in the water, and the sand in the water but not dissolved in the water (the sand will not dissolve because sand is insoluble).
The next step is to filter the mixture, which we do by pouring the mixture through a funnel that is lined with filter paper. The water and the dissolved salt will pass through the filter paper, but the insoluble sand will not – the sand will be left on the filter paper (the sand will be the residue). We then collect the sand off of the filter paper.
The solution that has passed through the filter paper (the filtrate) is water with dissolved salt. We can separate the salt from the water by using evaporation. We pour the filtrate into an evaporating dish and heat it up using a Bunsen burner. The heat will cause the water to evaporate, which will leave us with salt crystals. Instead of evaporation, we could have used crystallisation to obtain the salt crystals (the salt in rock salt won’t break down or react when heat is applied, but if the salt did break down or react when heat is applied, we would have had to use crystallisation instead of evaporation).
We now have separated the sand and salt in rock salt.