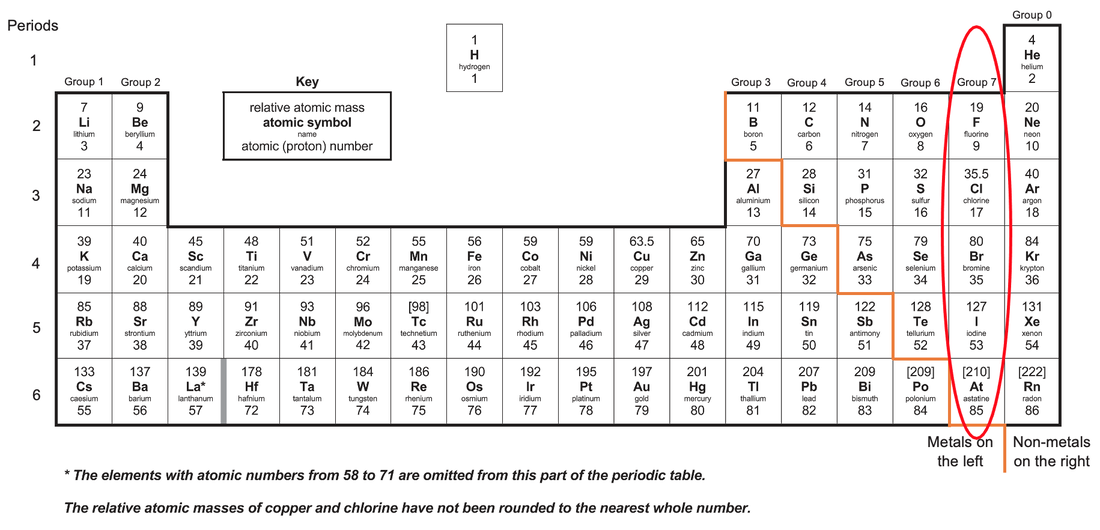
C1 N) Group 7 Elements
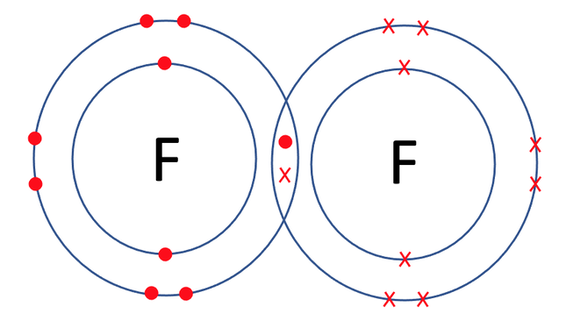
Halogens travel around as diatomic molecules, which means that they travel around in pairs of the same element. For example, they travel around as F2, Cl2, Br2 etc. The two atoms form a single covalent bond. A covalent bond is where two non-metals share pairs of electrons with each other. As both of the halogens want to gain 1 electron, they each put one electron in the sharing section (the crossover of the two atoms). The dot and cross diagram for the covalent bonding in F2 is shown below.
In addition to covalent bonds, halogens can also form ionic bonds. Ionic bonds involve a metal and a non-metal. In ionic bonding, the metal gives electron(s) to the non-metal. When halogens form ionic bonds, the halogen gains one electron, which results in them having a full outer shell. As the halogens gain one electron, they will become negatively charged and have a charge of 1-. We call negatively charged halogen ions halides; examples of halides are F-, Cl-, Br-, I- and At-.
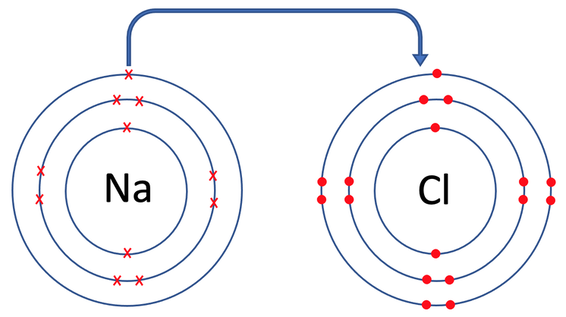
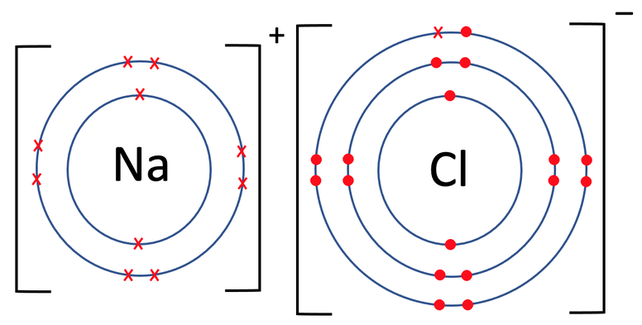
Sodium chloride is an example of an ionic compound. Sodium is in group 1, which means that it has one electron in its outermost electron shell; it will want to lose this electron to obtain a full outer shell. Chlorine is in group 7, which means that it has 7 electrons in its outermost electron shell; it will want to gain one electron to obtain a full outer shell. During the ionic bonding, the sodium atom gives one electron to the chlorine atom. As sodium has given one electron, it will have a charge of 1+. As chlorine has gained one electron, it will have a charge of 1-. Both sodium and chlorine now have full outer shells and are therefore happy. The bonding of sodium chloride is shown below, and on the diagram, you can see the electron that sodium has given to chlorine (the top diagram is the atoms prebonding, and the bottom diagram is the atoms post bonding).
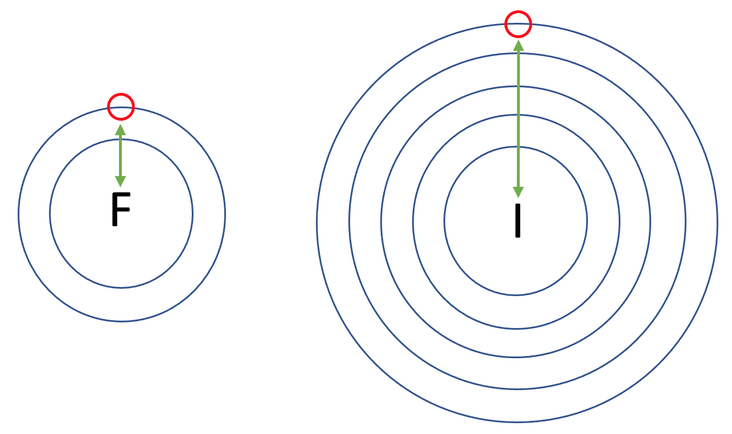
The reactivity of halogens decreases as we go down the group 7 column; fluorine is the most reactive, then chlorine, bromine, … and astatine is the least reactive. This is because higher up group 7 elements are trying to gain an electron that is on a shell that is closer to the positively charged nucleus. This means that there is a stronger force of attraction for higher up group 7 elements between the positively charged nucleus and the electron that the group 7 element is trying to gain. This stronger force of attraction means that higher up group 7 elements can attract an electron more easily, thus meaning that the group 7 elements at the top are the most reactive (the reactivity of group 7 elements decreases as we go down the periodic table).
For example, let’s have a look at fluorine and iodine; both of these want to gain one electron. Fluorine is in period 2 so wants to gain an electron on its second shell. Iodine is in period 5 so wants to gain an electron on its fifth shell. The diagram below shows the location of the electron that both fluorine and iodine want to gain.
Halogens have low melting and boiling points. This is because halogens have weak intermolecular forces of attraction (the attraction between each of the halogen molecules), which requires little energy to be broken, hence the low melting and boiling points. The melting and boiling points for halogens increase as we move down the group 7 column. This is because the relative atomic/ molecular masses increase as we go down the group 7 column, which increases the intermolecular forces of attraction, which requires more energy to break, hence the higher melting and boiling points for halogens that are further down the group 7 column.
All of the halogens are coloured vapours. Here are the characteristics of each of them (it is worth getting the characteristics below down on a revision card):
- Fluorine is a poisonous yellow gas (it is also the most reactive)
- Chlorine is a poisonous dense green gas (used to sterilise water – e.g. swimming pools)
- Bromine is a poisonous dense red-brown-orange liquid (used to make pesticides and plastics)
- Iodine is a grey solid or purple vapour (used to sterilise wounds)