Back to C1 Home
C1 P) Group 0 Elements
C1 P) Group 0 Elements
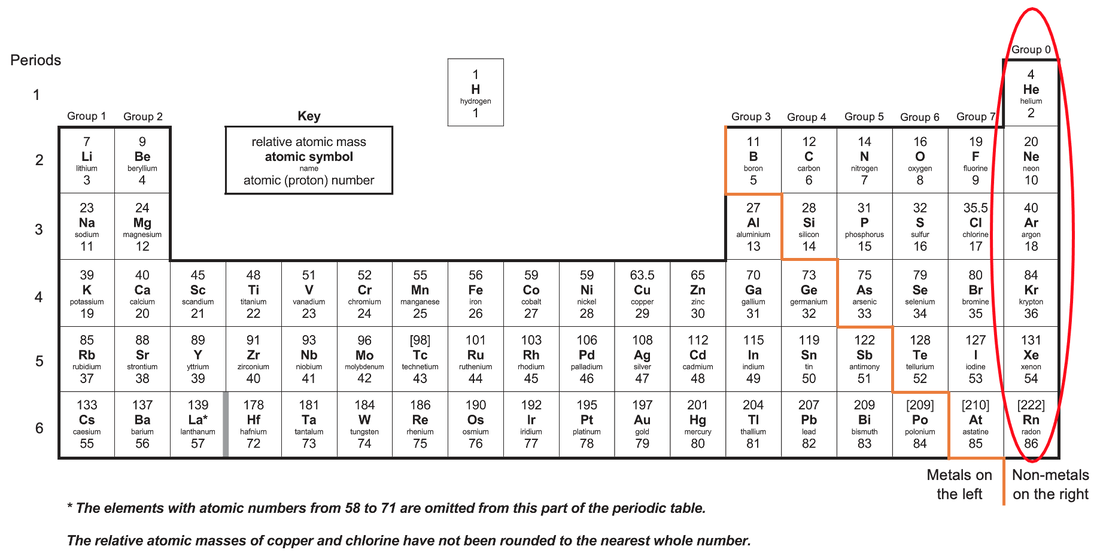
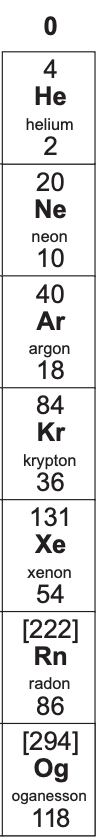
Group 0 elements are in the far-right column of the periodic table and they are known as the noble gases. The noble gases are helium, neon, argon, krypton, xenon and radon.
All of the group 0 elements have full outer electron shells; they all have 8 electrons in their outermost shells except helium which has 2 electrons in its outermost shell (helium has only 1 shell and the first shell only takes a maximum of 2 electrons).
Noble gases are stable and unreactive (or inert) because they have full outer shells of electrons (a stable arrangement of electrons). This means that noble gases do not easily give up, gain or share electron(s) easily. Noble gases are non-flammable (they do not catch fire easily). They are all colourless gases at room temperature. They are also monatomic, which means that they travel around as single atoms.
Noble gases are stable and unreactive (or inert) because they have full outer shells of electrons (a stable arrangement of electrons). This means that noble gases do not easily give up, gain or share electron(s) easily. Noble gases are non-flammable (they do not catch fire easily). They are all colourless gases at room temperature. They are also monatomic, which means that they travel around as single atoms.
Characteristics of Noble Gases
The densities, melting points and boiling points of noble gases increase as you go down the group 0 elements in the periodic table. This happens because as we go down the periodic table, the atoms become larger (they have higher relative atomic masses), which results in stronger intermolecular forces of attraction. The stronger intermolecular forces of attraction require more energy to break, thus meaning that as we go down the group 0 elements, the melting points and boiling points increase.
Helium is at the top of the group 0 elements in the periodic table and helium has the lowest density, lowest melting point and lowest boiling point. Radon is the furthest down group 0 element and it has the highest density, highest melting point and highest boiling point.
The densities, melting points and boiling points of noble gases increase as you go down the group 0 elements in the periodic table. This happens because as we go down the periodic table, the atoms become larger (they have higher relative atomic masses), which results in stronger intermolecular forces of attraction. The stronger intermolecular forces of attraction require more energy to break, thus meaning that as we go down the group 0 elements, the melting points and boiling points increase.
Helium is at the top of the group 0 elements in the periodic table and helium has the lowest density, lowest melting point and lowest boiling point. Radon is the furthest down group 0 element and it has the highest density, highest melting point and highest boiling point.
Predicting Characteristics
In the exam, you may be given the characteristics of some noble gases and asked to predict the characteristics of another noble gas. The characteristics may be to do with the melting and boiling points or densities. We answer these types of questions by using the rules for group 0 elements.
Example 1
The melting point of helium is -272°C and the melting point of argon is -189°C. Estimate the melting point for Neon.
Before we answer this question, I am going to put down the periodic table for the group 0 elements.
In the exam, you may be given the characteristics of some noble gases and asked to predict the characteristics of another noble gas. The characteristics may be to do with the melting and boiling points or densities. We answer these types of questions by using the rules for group 0 elements.
Example 1
The melting point of helium is -272°C and the melting point of argon is -189°C. Estimate the melting point for Neon.
Before we answer this question, I am going to put down the periodic table for the group 0 elements.
From the periodic table, we can see that neon (which we are predicting the properties of) is in the middle of helium and argon. We know that as we go down the periodic table for group 0 elements, the melting points, boiling points and densities increase. This means that as neon is in the middle of helium and argon, it will have a melting point that is in between helium’s and argon’s melting point. Therefore, we can predict the melting point of neon by finding the midpoint between the melting points for helium and argon. We find the midpoint by adding both of the melting points together and dividing by 2 (we essentially use the same method as finding the mean).
Our estimate for the melting point of neon is -230.5°C. The actual melting point for neon is -249°C, so we were not too far off.
In the exam, there will be a range of acceptable answers for your estimates of melting points, boiling points and densities.
In the exam, there will be a range of acceptable answers for your estimates of melting points, boiling points and densities.
Other Predictions
We may be given the densities of two group 0 elements and asked to predict the density of another group 0 element; the two group 0 elements that we will be given the densities of will be either side of the group 0 element that we are being asked to predict the density of. Our prediction for the density of the group 0 element will be the midpoint between the densities of the two group 0 elements that we were given. We find the midpoint by adding the two densities up and dividing by 2.
We may also be asked to predict characteristics about group 1 and group 7 elements. We answer these types of questions in pretty much the same way.
We may be given the densities of two group 0 elements and asked to predict the density of another group 0 element; the two group 0 elements that we will be given the densities of will be either side of the group 0 element that we are being asked to predict the density of. Our prediction for the density of the group 0 element will be the midpoint between the densities of the two group 0 elements that we were given. We find the midpoint by adding the two densities up and dividing by 2.
We may also be asked to predict characteristics about group 1 and group 7 elements. We answer these types of questions in pretty much the same way.
Uses of Noble Gases
Here are some of the uses of noble gases:
Here are some of the uses of noble gases:
- Helium is used as a lifting gas as it is less dense than air. It is used in balloons for celebrations (birthday parties) and in air ships. It is also non-flammable.
- Argon is used in filament bulbs because it is inert (it is usually argon, but other noble gases can be used). When a filament bulb is on, the metal wire (the filament) gets very hot. If air was used inside the bulb, the wire would burn away/ react with the oxygen in the air in the bulb resulting in the bulb breaking. This does not happen when the filament bulb is filled with argon because the argon will not react with the wire in the bulb, which means that the wire does not burn away and therefore keeps working. Noble gases are also used in flash photograph for the same reason – they are unreactive, so prevent the wire from burning away/ reacting with oxygen during the high temperatures when a flash is on.
- Argon and helium are used to protect metals when they are being welded (joined together). They are used because they are inert and therefore will not react with the hot metal. If the metal was welded in air, the hot metal will react with the oxygen in air resulting in a metal oxide forming.