Back to C1 Home
C1: Quiz 13
C1: Quiz 13
1) What is another name for the group 7 elements?
2)
a) How many electrons do the group 7 elements have in their outermost shell?
b) How many electrons are the group 7 elements off having a full outer shell?
3)
a) Halogens travel around as diatomic molecules; what does that mean?
b) What are the bonds involved in the diatomic molecules?
4)
a) With respect to all of the other elements in the periodic table, do all group 7 elements have relatively high or relatively low melting and boiling points? Explain your answer.
b) Do the melting and boiling points of the halogens increase or decrease as we go down the group 7 column? Explain your answer.
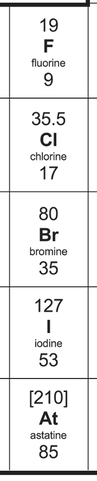
5) The halogens in the periodic table are shown below.
2)
a) How many electrons do the group 7 elements have in their outermost shell?
b) How many electrons are the group 7 elements off having a full outer shell?
3)
a) Halogens travel around as diatomic molecules; what does that mean?
b) What are the bonds involved in the diatomic molecules?
4)
a) With respect to all of the other elements in the periodic table, do all group 7 elements have relatively high or relatively low melting and boiling points? Explain your answer.
b) Do the melting and boiling points of the halogens increase or decrease as we go down the group 7 column? Explain your answer.
5) The halogens in the periodic table are shown below.
Describe the reactivity trend for the halogens. Explain why the halogens have this reactivity trend.
6) Halogens can react with metals to produce halide ions. What would be the charge on a fluoride ion and how would this charge come about?
7) What colours and states are the following halogens at room temperature?
a) Fluorine
b) Chlorine
c) Bromine
d) Iodine
6) Halogens can react with metals to produce halide ions. What would be the charge on a fluoride ion and how would this charge come about?
7) What colours and states are the following halogens at room temperature?
a) Fluorine
b) Chlorine
c) Bromine
d) Iodine