Back to C1 Home
C1: Quiz 3 – Answers
C1: Quiz 3 – Answers
1) Isotopes have the same number of protons and a different number of neutrons
Or, isotopes have the same atomic number and a different relative atomic mass
2)
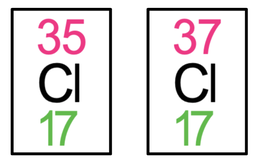
a) Chlorine-35: protons = 17, neutrons = 18
Chlorine-37: protons = 17, neutrons = 20
b) They both have 17 protons
c) They have a different number of neutrons – chlorine-35 has 18 neutrons and chlorine-37 has 20 neutrons.
Or chlorine-35 has 2 fewer neutrons than chlorine-37
Or chlorine-37 has 2 more neutrons than chlorine-35
Or anything like that
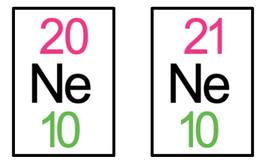
3) They have a different number of neutrons – neon-20 has 10 neutrons and neon-21 has 11 neutrons
Or neon-21 has 1 more neutron than neon-20
Or neon-20 has 1 fewer neutron than neon-21
4) 35.5
5) 10.8
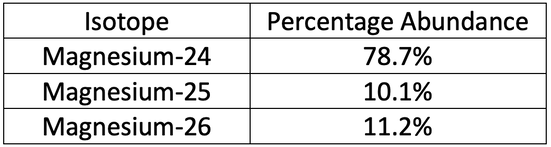
6) 24.325
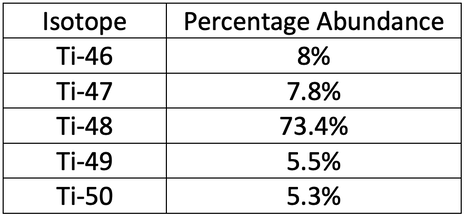
7) 47.923
8)
a)
Or, isotopes have the same atomic number and a different relative atomic mass
2)
a) Chlorine-35: protons = 17, neutrons = 18
Chlorine-37: protons = 17, neutrons = 20
b) They both have 17 protons
c) They have a different number of neutrons – chlorine-35 has 18 neutrons and chlorine-37 has 20 neutrons.
Or chlorine-35 has 2 fewer neutrons than chlorine-37
Or chlorine-37 has 2 more neutrons than chlorine-35
Or anything like that
3) They have a different number of neutrons – neon-20 has 10 neutrons and neon-21 has 11 neutrons
Or neon-21 has 1 more neutron than neon-20
Or neon-20 has 1 fewer neutron than neon-21
4) 35.5
5) 10.8
6) 24.325
7) 47.923
8)
a)
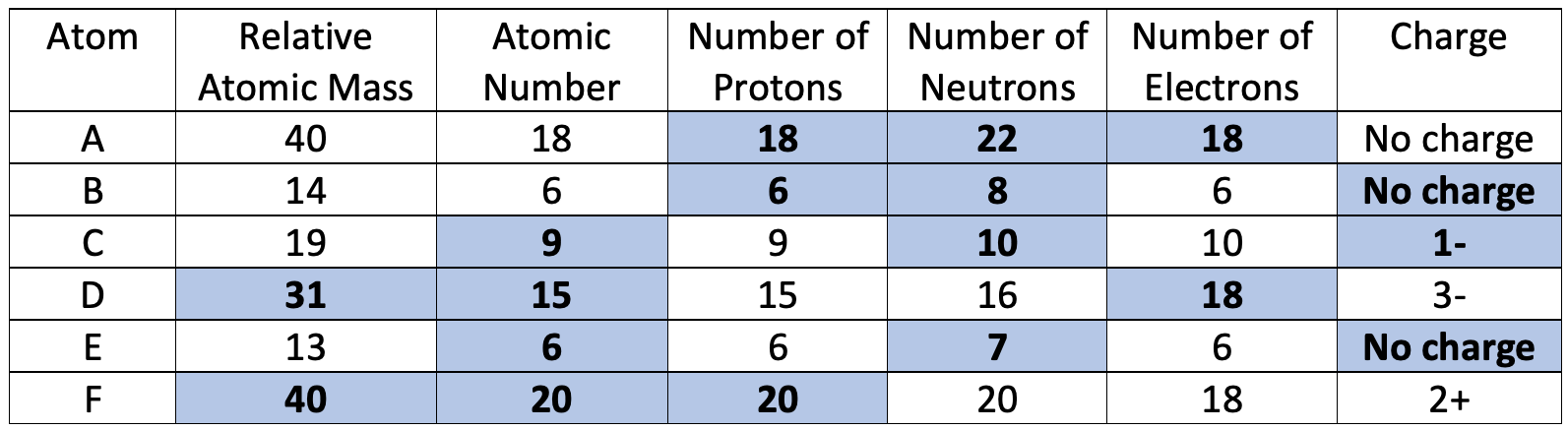
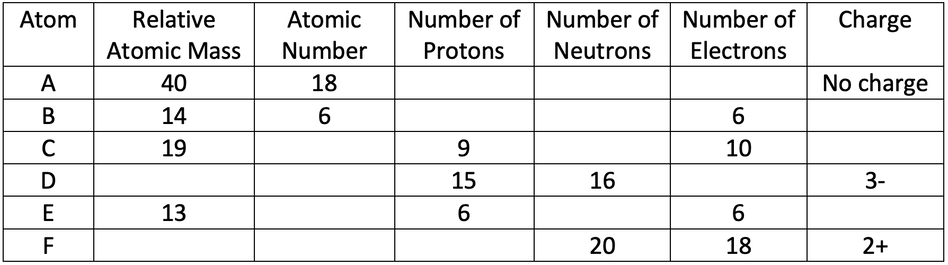
b) B and E are isotopes of each other as they have the same number of protons (they both have 6) and a different number of neutrons/ a different relative atomic mass (B has 8 neutrons/ an atomic mass of 14, and E has 7 neutrons/ an atomic mass of 13)
Questions
1) What is an isotope?
2) The tiles for chlorine-35 and chlorine-37 are both shown below.
1) What is an isotope?
2) The tiles for chlorine-35 and chlorine-37 are both shown below.
a) Find the number of protons and neutrons for both chlorine-35 and chlorine-37.
b) What is a similarity between chlorine-35 and chlorine-37?
c) What is a difference between chlorine-35 and chlorine-37?
3) The tiles for neon-20 and neon-21 are both shown below.
b) What is a similarity between chlorine-35 and chlorine-37?
c) What is a difference between chlorine-35 and chlorine-37?
3) The tiles for neon-20 and neon-21 are both shown below.
What is the difference between the structure of neon-20 and the structure of neon-21?
4) Chlorine has two isotopes; chlorine-35 has an abundance of 75%, and chlorine-37 has an abundance of 25%. Calculate the relative atomic mass for chlorine. Give your answer to one decimal place.
5) A sample of boron contains two different boron isotopes. In this boron sample, boron-10 has an abundance of 20%, and boron-11 has an abundance of 80%. Calculate the relative atomic mass of boron for this sample.
6) A sample of magnesium contains three isotopes. The isotopes and abundances are shown in the table below.
4) Chlorine has two isotopes; chlorine-35 has an abundance of 75%, and chlorine-37 has an abundance of 25%. Calculate the relative atomic mass for chlorine. Give your answer to one decimal place.
5) A sample of boron contains two different boron isotopes. In this boron sample, boron-10 has an abundance of 20%, and boron-11 has an abundance of 80%. Calculate the relative atomic mass of boron for this sample.
6) A sample of magnesium contains three isotopes. The isotopes and abundances are shown in the table below.
Calculate the relative atomic mass of magnesium for this sample. Do not round your answer.
7) A sample of titanium contains five isotopes. The isotopes and abundances are shown in the table below.
7) A sample of titanium contains five isotopes. The isotopes and abundances are shown in the table below.
Calculate the relative atomic mass of titanium for this sample. Do not round your answer.
8) The table below shows some information about 6 different atoms.
8) The table below shows some information about 6 different atoms.
a) Find the missing values in the table.
b) Which two in the table are isotopes of each other?
b) Which two in the table are isotopes of each other?