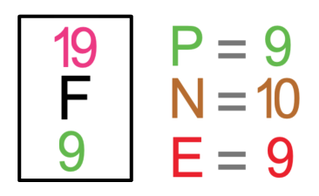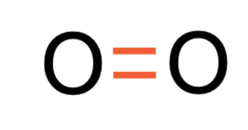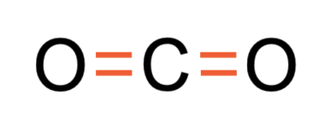C2 D) Covalent Bonds
Simple molecular substances are molecules that are made up of a few atoms joined together by covalent bonds. We are going to look at drawing some of these simple molecular substances in this section.
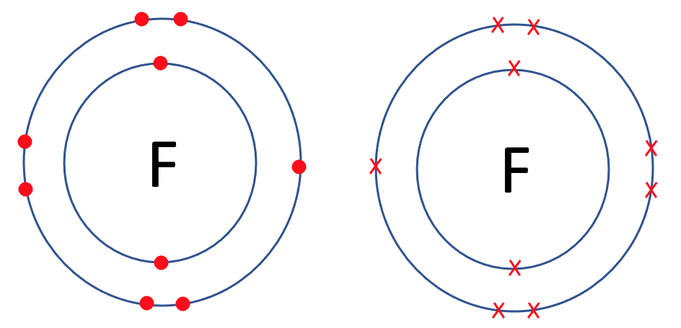
Fluorine travels around in the form of F2 (it is a diatomic molecule; travels around in pairs). The tile in the periodic table for fluorine is shown below along with the number of protons, neutrons and electrons.
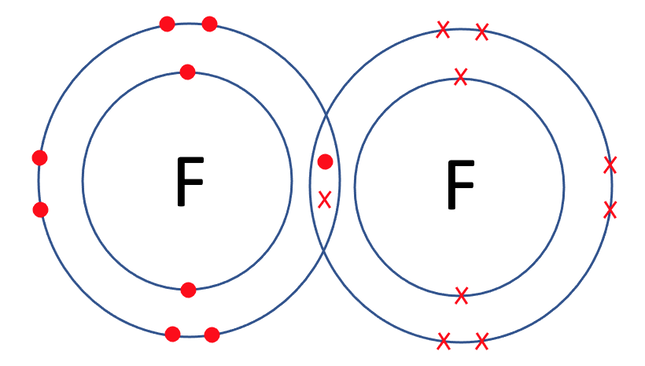
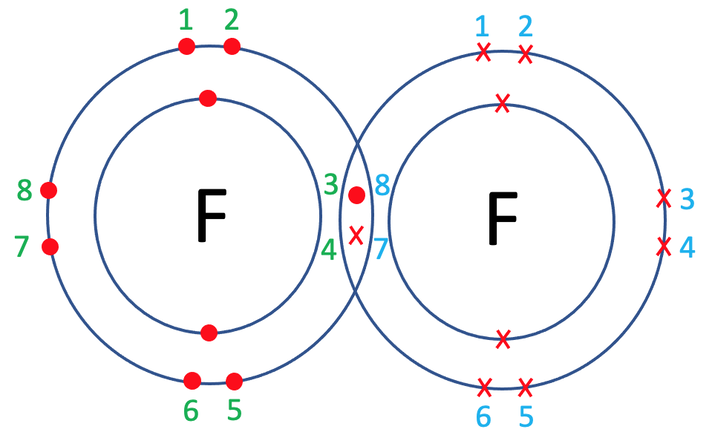
As there is only 1 pair of shared electrons, the fluorine atoms have formed a single covalent bond. We represent a single covalent bond with a single line (–). The displayed formula for F2 is shown below.
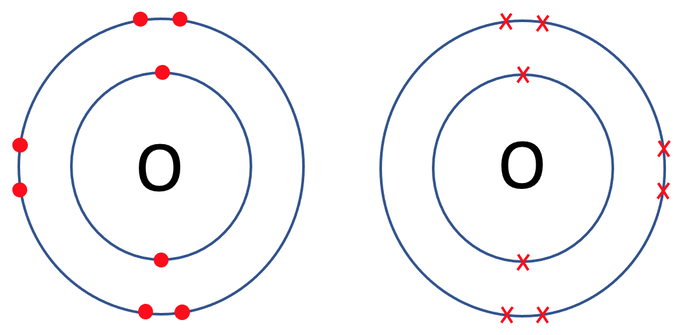
Oxygen travels around in the form of O2 (it is a diatomic molecule). The tile in the periodic table for oxygen is shown below along with the number of protons, neutrons and electrons.
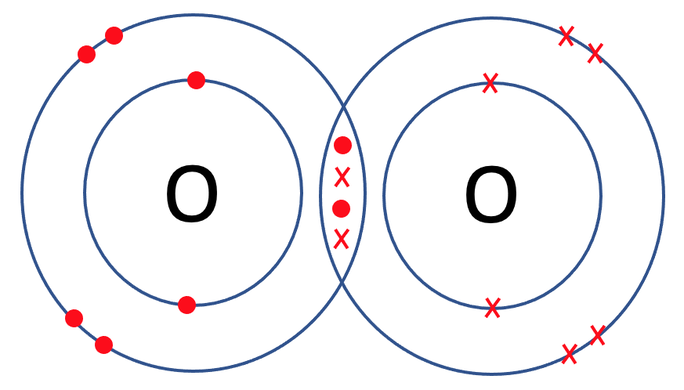
We can check that both of the oxygen atoms are happy by counting the electrons in the outer shells of the oxygen atoms to see if the shells are full. The outer shell for the oxygen atoms is the second shell, which takes a maximum of 8 electrons. When we count the electrons on the outer shells for both of the oxygen atoms, we see that they both have 8 electrons and are therefore happy.
The oxygens shared 2 pairs of electrons and this means that we have a double covalent bond. We represent a double covalent bond with a double line (=). The displayed formula for O2 is shown below.
Sometimes you will be asked to just draw the outmost electron shells for the atoms rather than all of the electron shells. This is because the inner electron shells can make the diagrams messy/ less clear. We are going to have a look at an example where this is the case.
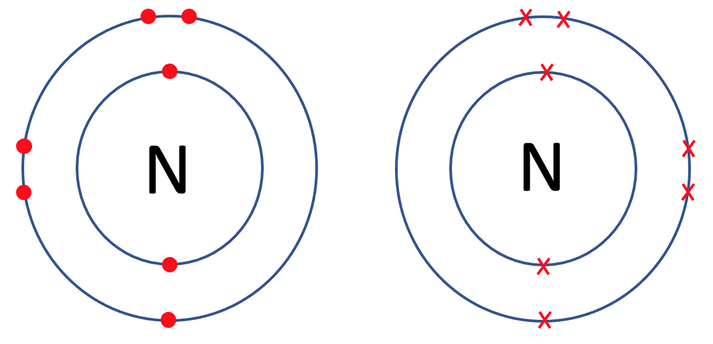
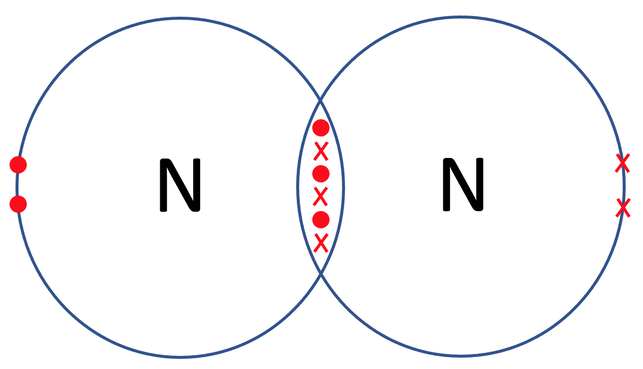
Nitrogen travels around in the form of N2 (it is a diatomic molecule). The tile in the periodic table for nitrogen is shown below along with the number of protons, neutrons and electrons.
We can check that both of the nitrogen atoms are happy by counting the electrons on each of their outer shells. Their outer shells are the second shell which takes a maximum of 8 electrons. When we count electrons, we see that they both have 8 electrons in their outer shells and are therefore happy.
The nitrogen atoms share 3 pairs of electrons and this means that we have a triple covalent bond. We represent a triple covalent bond with 3 lines (≡). The displayed formula for N2 is shown below.
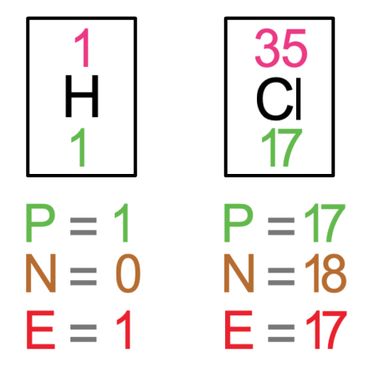
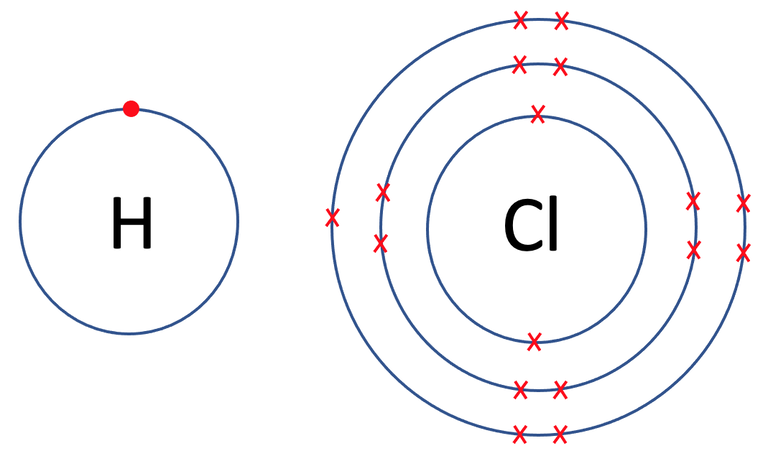
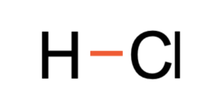
All of the previous examples have involved the same elements. We are now going to have a look at a few examples where the atoms involved are different elements. The first example that we are going to look at is hydrogen chloride (HCl). The tiles in the periodic table and the number of protons, neutrons and electrons for hydrogen and chlorine are shown below.
The outer shell for chlorine is its third shell and the third shell takes a maximum of 8 electrons. Currently, there are 7 electrons in its outer shell, which means that it needs to gain 1 electron in order to have a full outer shell.
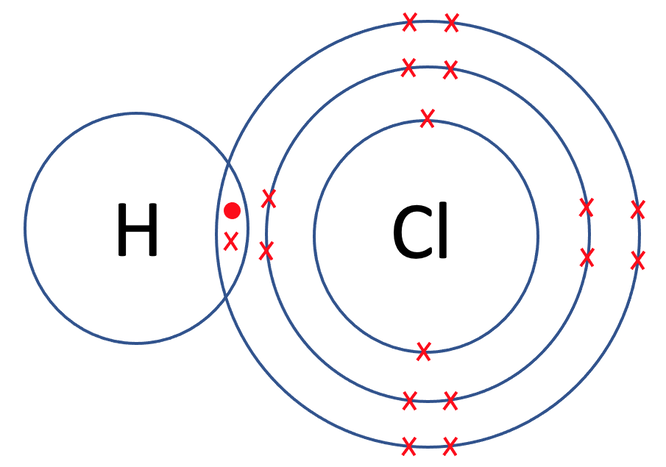
As both of these atoms are 1 off having a full outer shell, they share one pair of electrons; both of the atoms put 1 electron into the sharing part. The diagram for the bonding is shown below.
The hydrogen and chlorine shared 1 pair of electrons, which means that they formed 1 covalent bond. The displayed formula for HCl is shown below.
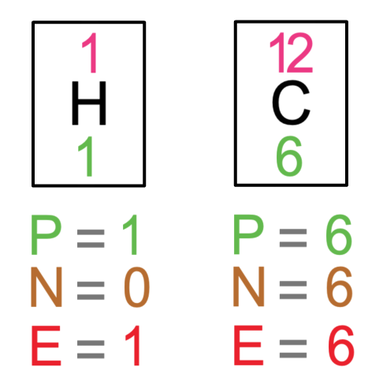
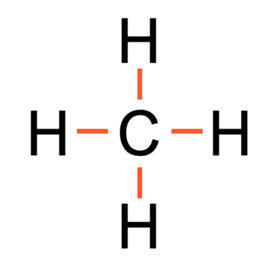
The next example that we are going to look at is methane, which is CH4. Methane is made out of 1 carbon atom and 4 hydrogen atoms. The tiles in the periodic table and the number of protons, neutrons and electrons for hydrogen and carbon are shown below (I have only drawn 1 hydrogen atom and not all 4).
Each of the hydrogens has 1 electron in its outer shell, which is the first shell. The first shell can take a maximum of 2 electrons and this means that each of the hydrogen atoms need to gain 1 electron in order to have a full outer shell.
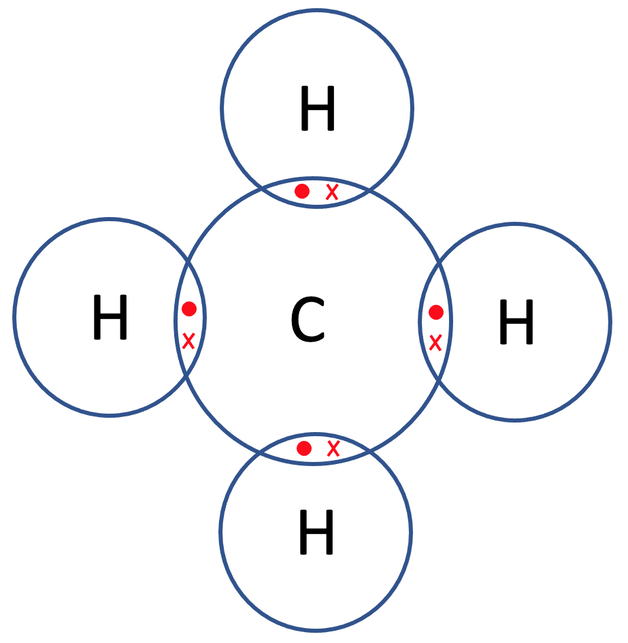
So, carbon needs to gain 4 electrons and all of the 4 hydrogens need to gain 1 electron. All of the atoms can obtain full outer shells by all of the hydrogen atoms sharing 1 electron with the carbon, and carbon sharing 1 electron with each of the 4 hydrogen atoms. The diagram of the bonding for methane with only the outer shells drawn is shown below.
Let’s now check that all of the atoms involved have full outer shells. The outer shell for all of the hydrogen atoms is the first shell, which takes a maximum of 2 electrons, and all 4 of the hydrogen atoms have 2 electrons in their outer shells. The outer shell for carbon is the second shell, which takes a maximum of 8 electrons, and carbon on our diagram does have 8 electrons in its outer shell. All of the 5 atoms involved have full outer shells and are therefore happy.
All of the bonds in the above diagram are single covalent bonds as there is only 1 pair of shared electrons in each of the overlapping parts of the shells. The displayed formula for CH4 is shown below.
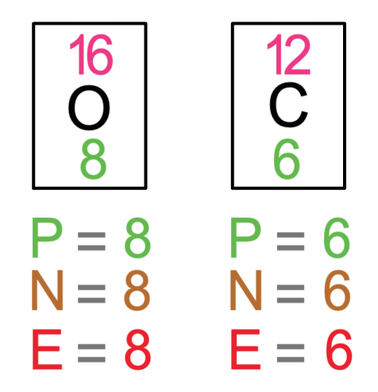
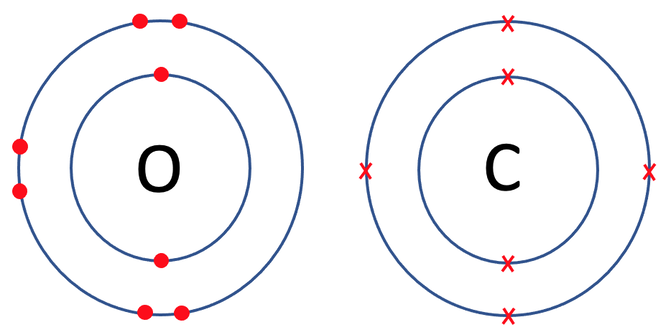
The final example that we are going to look at is carbon dioxide, which is CO2. There is 1 carbon atom and 2 oxygen atoms in carbon dioxide. The tiles in the periodic table and the number of protons, neutrons and electrons for carbon and oxygen are shown below.
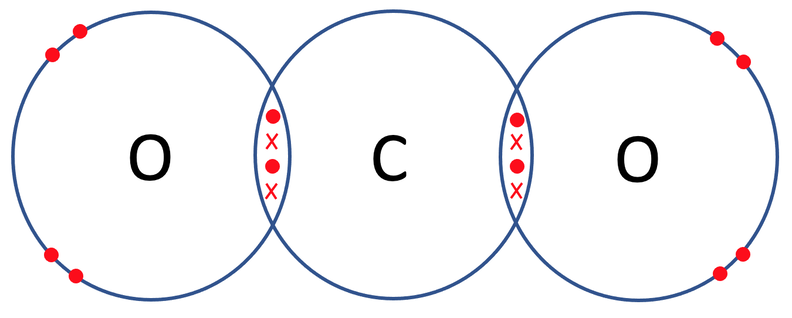
So, carbon needs to gain 4 electrons and both of the oxygen atoms need to gain 2 electrons. All of the atoms can obtain full outer shells by carbon sharing 2 electrons with each of the oxygens, and each of the oxygens sharing 2 electrons with carbon. The diagram of the bonding for carbon dioxide with only the outer shells drawn is shown below.
Let’s now check that all of the atoms involved have full outer shells. The outer shell for all of the atoms is the second shell, which takes a maximum of 8 electrons. When we count the electrons in each of the outer shells, we see that they all have 8 electrons, thus meaning that the outer shells are full and the atoms are happy.
For both of the bonds in carbon dioxide, there are 2 pairs of shared electrons. This means that both of the bonds are double covalent bonds; we represent this with a = in the displayed formula. The displayed formula for CO2 is shown below.
We are now going to have a look at the positives and negatives of dot & cross diagrams (dot & cross diagrams are the diagrams that we have looked at in this section). A positive of dot & cross diagrams is that they are good at showing which atoms the electrons come from. A negative of dot & cross diagrams is that they do not show the relative sizes of the atoms.