C2 E) Simple Molecular Substances
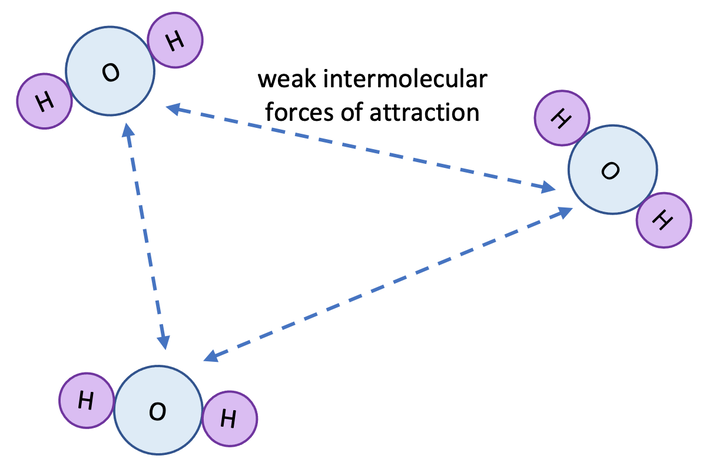
However, the forces of attraction between the molecules in a simple molecular substance are very weak; we say that the intermolecular forces of attraction are weak. For the water example, each of the water molecules are not very attracted to each other. The intermolecular forces of attraction are shown as dotted lines on the diagram below.
Not much energy is required to break these weak intermolecular forces of attraction and this is why simple molecular substances have low melting and boiling points. Most simple molecular substances are gases or liquids at room temperature. For example, oxygen (O2) is a gas at room temperature, carbon dioxide (CO2) is a gas at room temperature and water (H2O) is a liquid at room temperature.
As the simple molecular substances get larger (contain more bonds), the strength of the intermolecular forces of attraction increase, which means that more energy is required to break the stronger intermolecular forces of attraction, thus meaning that larger simple molecular substances have higher melting and boiling points. For example, the boiling point of pentane (C5H12) is 36°C and the boiling point of decane (C10H22) is 174°C [pentane and decane are both hydrocarbons that come from crude oil].
Simple molecular substances do not conduct electricity in all states. This is because there are no delocalised electrons or ions in simple molecular substances.