Back to C2 Home
C2: Quiz 5
C2: Quiz 5
1) Fill in the passage below using the words “strong” and “weak”.
Simple molecular substances have extremely ________ covalent bonds that join all of the atoms within a simple molecular substance together. The intermolecular forces of attraction for simple molecular substances are very ________.
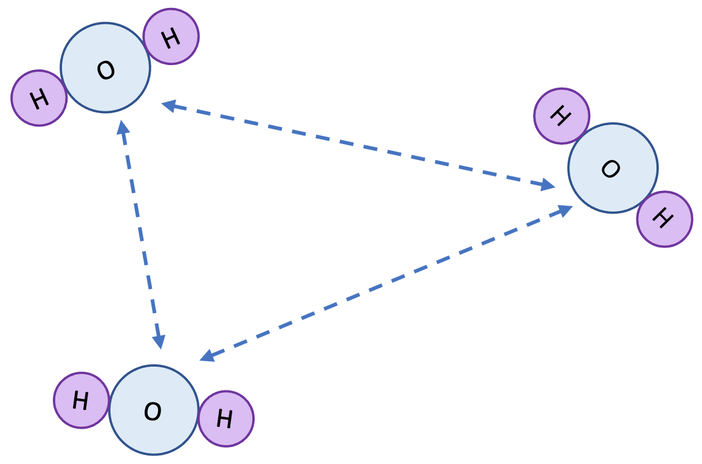
2) What do the dashed lines on the diagram below represent?
Simple molecular substances have extremely ________ covalent bonds that join all of the atoms within a simple molecular substance together. The intermolecular forces of attraction for simple molecular substances are very ________.
2) What do the dashed lines on the diagram below represent?
3) Do simple molecular substances have high or low melting and boiling points? Explain your answer.
4) Do simple covalent substances conduct electricity? Explain your answer.
5) Do the melting and boiling points increase or decrease as the sizes of the simple molecular substances increase?
6) We have three hydrocarbons; methane (CH4), pentane (C5H12) and dodecane (C12H26).
a) Which substance has the highest melting and boiling point? Explain your answer.
b) Which substance has the lowest melting and boiling point? Explain your answer.