Back to C2 Home
C2: Quiz 8 – Answers
C2: Quiz 8 – Answers
1)
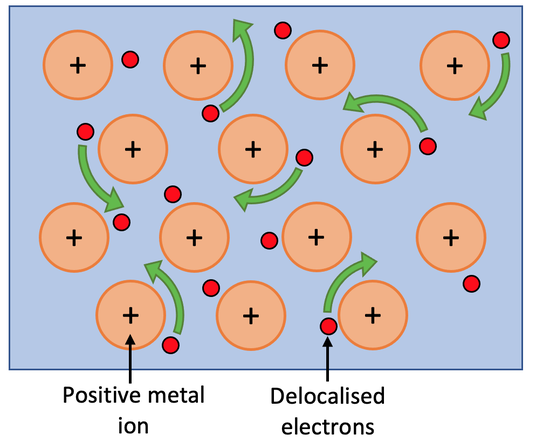
a) Metallic bonding is the strong force of attraction between the positive metal ions and the negative delocalised electrons
b)
a) Metallic bonding is the strong force of attraction between the positive metal ions and the negative delocalised electrons
b)
c) Metals have very high melting and boiling points because there is a very strong force of attraction between the positive metal ions and the negative delocalised electrons, which requires a lot of energy to break/ overcome, thus meaning that metallically bonded substances have very high melting and boiling points
d) They are good conductors of electricity because they have delocalised electrons that are free to move and carry a charge
e) They are good conductors of thermal energy because they have delocalised electrons that are free to move and carry thermal energy
2)
a)
d) They are good conductors of electricity because they have delocalised electrons that are free to move and carry a charge
e) They are good conductors of thermal energy because they have delocalised electrons that are free to move and carry thermal energy
2)
a)
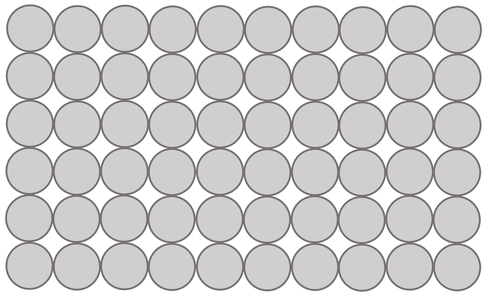
b) The atoms in pure metals have a regular layered structure/ arrangement. These layers will easily slide over each other when a force is applied, which is why pure metals are soft/ malleable
3)
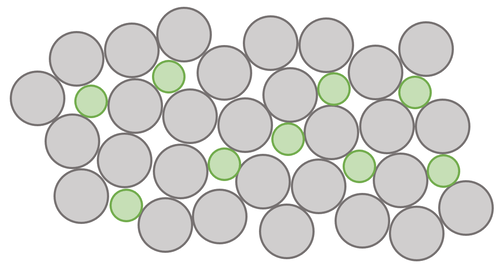
a) An alloy is a mixture of 2 or more elements where at least one of the elements is a metal
b)
3)
a) An alloy is a mixture of 2 or more elements where at least one of the elements is a metal
b)
c) Alloys are stronger than pure metals because the different sized atoms that are added disrupt the regular layers/ arrangement of atoms in the pure metals. This makes it harder for the layers to slide past each other, thus meaning that alloys are stronger than pure metals
Questions
1)
a) Give a definition for metallic bonding.
b) Draw a diagram for metallic bonding.
c) Do substances that are metallically bonded have very high or very low melting and boiling points? Explain your answer.
d) Are metallically bonded substances good conductors of electricity? Explain your answer.
e) Are metallically bonded substances good conductors of thermal energy? Explain your answer.
2)
a) Draw the arrangement of the atoms in a pure metal.
b) Pure metals are known as being malleable. Explain why.
3)
a) Give a definition of an alloy.
b) A particular alloy will be made out of 2 different elements. Draw the arrangement of the atoms/ elements in an alloy. Clearly show the different elements by shading one of the elements.
c) Why are alloys stronger than pure metals?
1)
a) Give a definition for metallic bonding.
b) Draw a diagram for metallic bonding.
c) Do substances that are metallically bonded have very high or very low melting and boiling points? Explain your answer.
d) Are metallically bonded substances good conductors of electricity? Explain your answer.
e) Are metallically bonded substances good conductors of thermal energy? Explain your answer.
2)
a) Draw the arrangement of the atoms in a pure metal.
b) Pure metals are known as being malleable. Explain why.
3)
a) Give a definition of an alloy.
b) A particular alloy will be made out of 2 different elements. Draw the arrangement of the atoms/ elements in an alloy. Clearly show the different elements by shading one of the elements.
c) Why are alloys stronger than pure metals?