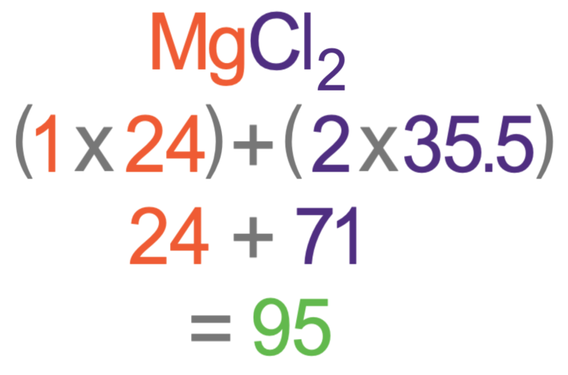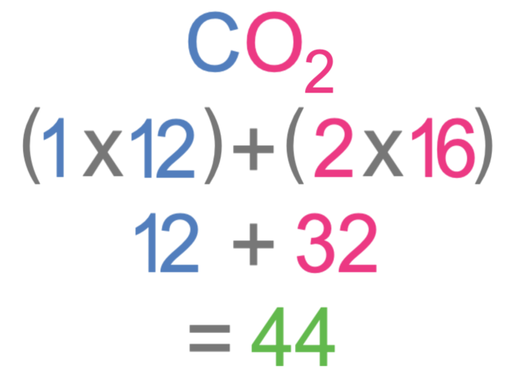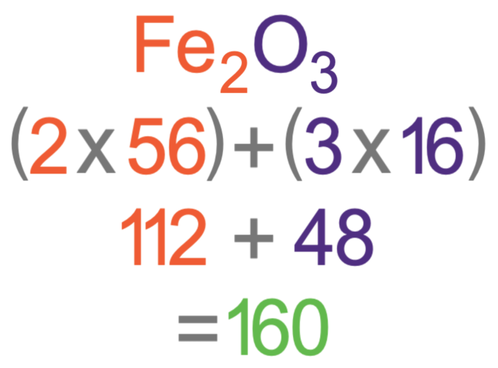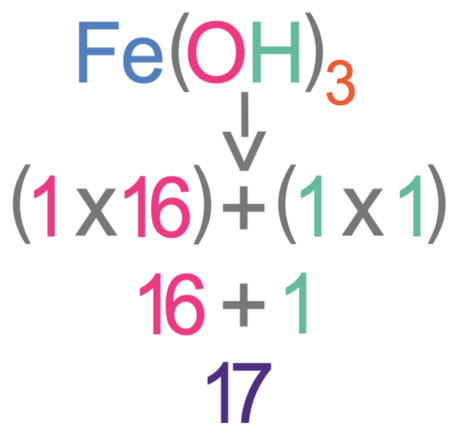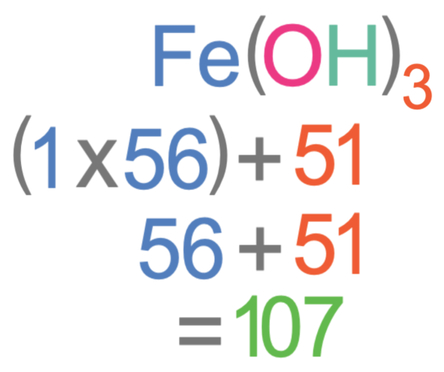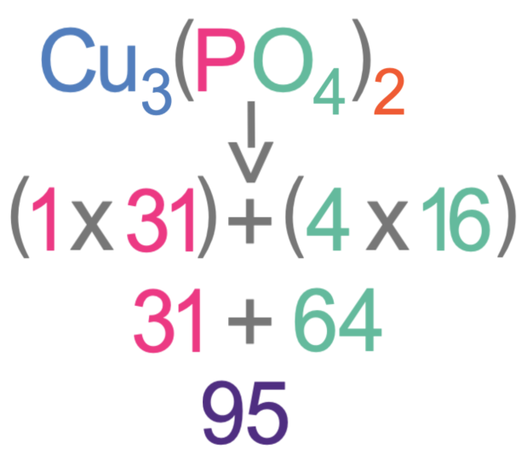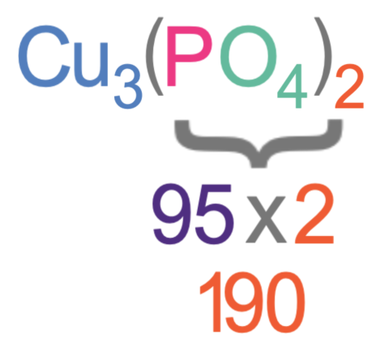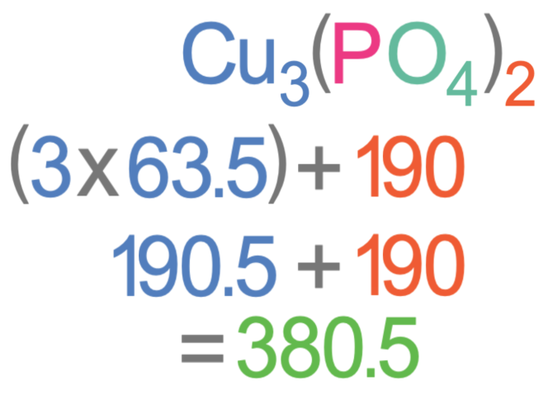C3 A) Relative Formula Mass
The relative formula mass is the relative mass of a molecule. The notation for relative formula mass is Mr. We work out the relative formula mass of a molecule by adding the relative atomic masses of all of the atoms that are involved in the molecule. The notation for the relative atomic mass (the mass of an atom) is Ar. Let’s have a look at a few examples.
Work out the relative formula mass of magnesium chloride (MgCl2).
The Ar of magnesium is 24 and the Ar of chlorine is 35.5
The relative formula mass for MgCl2 is 95. There are no units because this mass is relative.
What is the relative formula mass of carbon dioxide (CO2)?
The Ar of carbon (C) is 12 and the Ar of oxygen (O) is 16.
What is the relative formula mass of Fe2O3?
The Ar of iron (Fe) is 56 and the Ar of oxygen (O) is 16.
We answer this question in the same way as the previous two questions; we multiply the number of each of the different elements by the atomic mass for that element. We then add the outcomes up. In Fe2O3, there are two iron atoms and three oxygen atoms. The relative atomic mass of iron is 56 and the relative atomic mass of oxygen is 16. The working for finding the relative formula mass is shown below.
Sometimes it will be the case that we are asked to work out the relative formula mass for substances that involve brackets. The best way to answer these types of questions is to work out the mass of everything inside the bracket. We then multiply the mass of everything inside the bracket by the number that is outside the bracket. The final step is to add the mass of all of the atoms that are outside the bracket onto the mass of the bracket. This will all make more sense after we have looked at the next two examples.
Example 4
Iron (III) hydroxide has the chemical formula Fe(OH)3. Find the relative formula mass of iron (III) hydroxide.
The Ar of iron (Fe) is 56, the Ar of oxygen (O) is 16 and the Ar of hydrogen is 1.
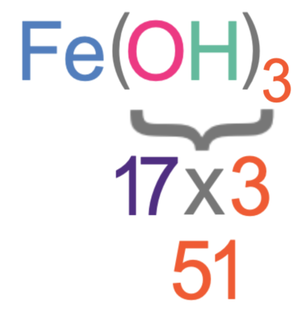
The subscript after the bracket is a 3, and this means that there are 3 lots of everything that is inside the bracket.
The first step in working out the relative formula mass of iron (III) hydroxide is to work out the mass of everything inside the bracket. Inside the bracket, there is one oxygen and one hydrogen. The Ar of oxygen is 16 and the Ar of hydrogen is 1.
The total mass of the bracket part of the molecule is 51.
We now need to add the masses of all of the atoms that are outside the brackets. There is one Fe outside the bracket and the Ar of Fe is 56. Therefore, we add 56 and the total mass of the brackets together.
The relative formula mass of Fe(OH)3 is 107.
Find the relative formula mass of Cu3(PO4)2.
The Ar of copper (Cu) is 63.5, the Ar of phosphorus (P) is 31 and the Ar of oxygen (O) is 16.
The 2 outside of the brackets means that there are 2 lots of everything that is inside the brackets.
The first step in working out the relative formula mass of this substance is to work out the mass of the bracket. Inside the bracket there is 1 phosphorous and 4 oxygens. The Ar of phosphorus is 31 and the Ar of oxygen is 16. The working for finding the mass inside the bracket is shown below.
The total mass from the brackets is 190.
We now need to add the masses of all of the atoms that are outside the brackets. Outside the brackets there are 3 copper atoms and the Ar of copper is 63.5. Therefore, we multiply 3 by 63.5 to work out the total mass of everything outside the bracket. We then add this onto the mass of everything inside the bracket.
The relative formula mass for Cu3(PO4)2 is 380.5.