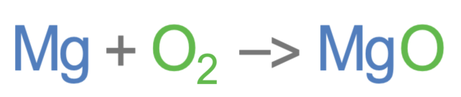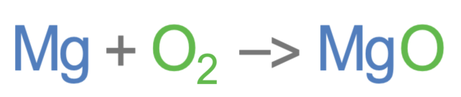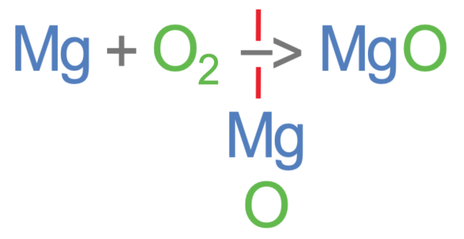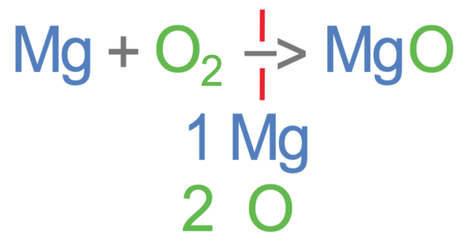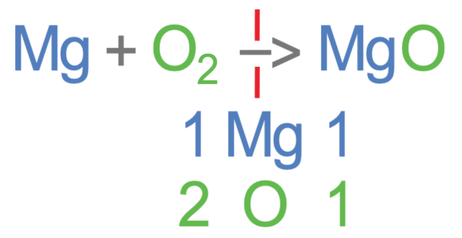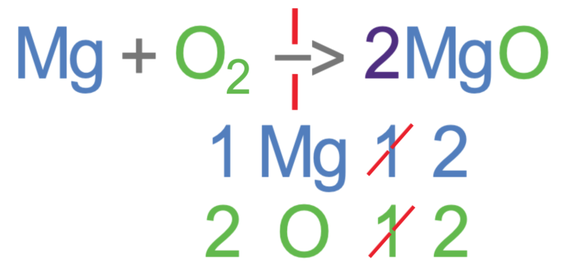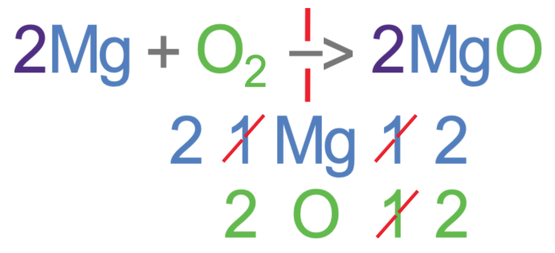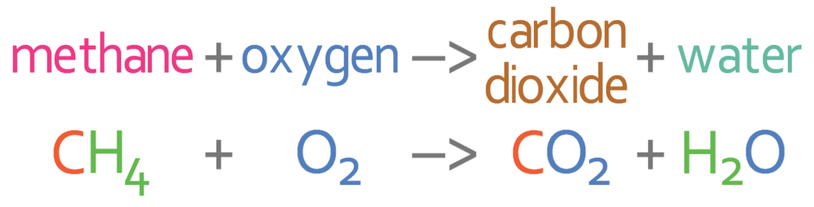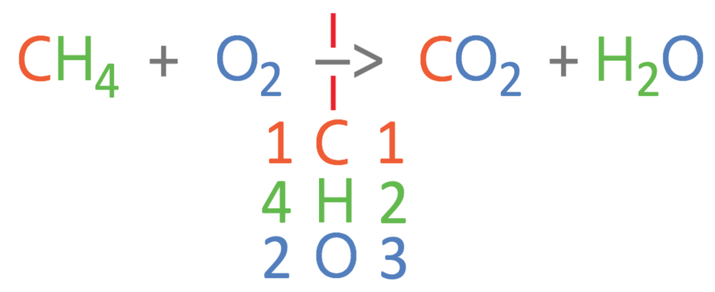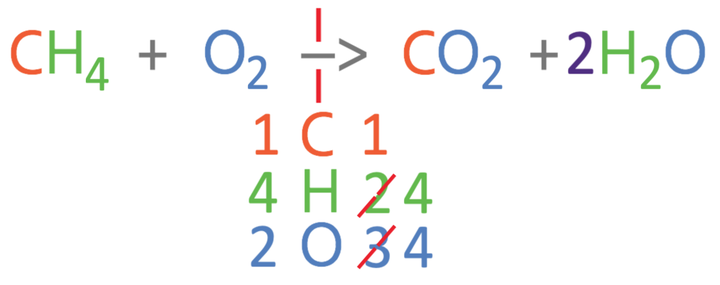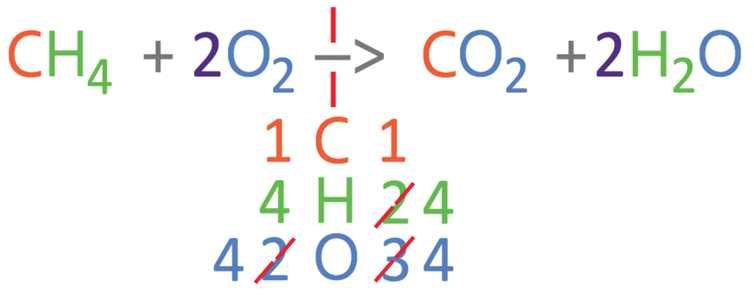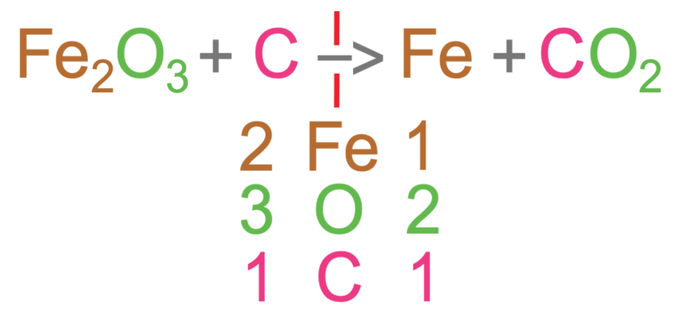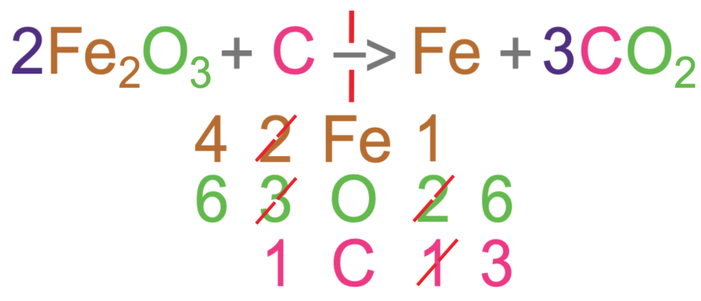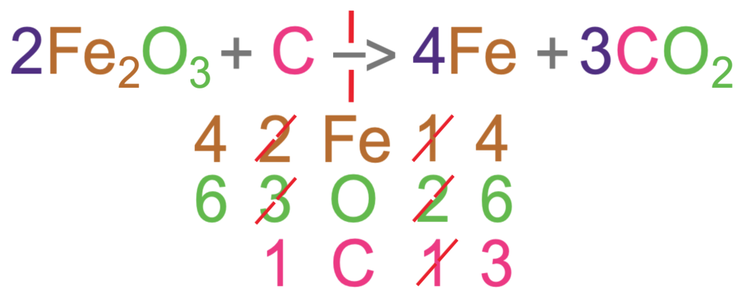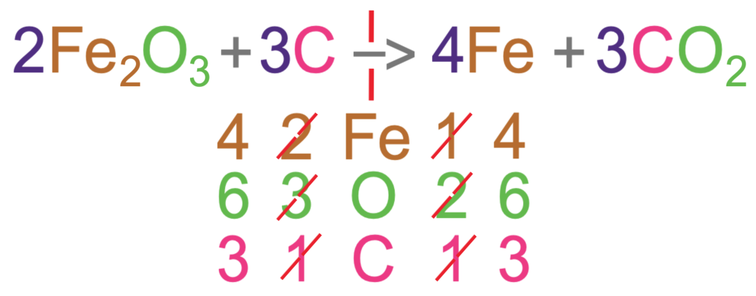C3 D) Balancing Equations
For example, we have the reaction below.
Let’s now look at how we balance chemical equations.
Magnesium is burnt in air (oxygen) to produce magnesium oxide. The unbalanced equation is shown below:
The first step in balancing a chemical equation is to write down the number of each of the different elements in the reactants and the products. It is best to write the different elements down the reaction arrow and write the number of that element in the reactants on the left and the number of that element in the products on the right.
Let’s now work out the number of the different elements on the left side of the equation (the reactants). In the reactants, there is 1 magnesium from the Mg, and 2 oxygens from the O2.
Whenever we add a big number in front of a molecule/ substance, we should always recount all of the different elements on the side that we have added the big number to. On the right side of the reaction, we have 2 magnesium oxides and for each magnesium oxide, there is 1 magnesium and 1 oxygen. Therefore, in the 2 magnesium oxides, there are 2 magnesiums and 2 oxygens.
Methane is burnt in air (oxygen) to produce carbon dioxide and water. The unbalanced equation is shown below.
The first step in balancing the equation is to work out how many of the different elements we have on each side of the arrow. There are 3 different elements in this reaction; carbon, hydrogen and oxygen. I am going to place these underneath the arrow and then work out the amount of the 3 different elements in the reactants and the products.
For the left side of the reaction, there is 1 carbon atom, 4 hydrogen atoms and 2 oxygen atoms.
For the right side of the reaction, there is 1 carbon atom, 2 hydrogen atoms and 3 oxygen atoms (2 from the CO2 and 1 from the H2O).
From comparing the numbers on the left and right, we can see that the carbons are balanced, but the hydrogens and oxygens are not balanced. I am going to balance the hydrogens first; currently there are 4 hydrogens on the left and 2 hydrogens on the right. We can make the hydrogens balance by having 2 waters (H2O) on the right, which will give us 4 hydrogen atoms on the right. As we are having 2 waters on the right, we place a big 2 in front of the water (2H2O). As we have made a modification to the right side of the reaction, we should count the number of all 3 elements again (it is always worth counting again to avoid making a mistake). On the right, there is 1 carbon atom, 4 hydrogen atoms and 4 oxygen atoms (2 from the CO2 and 2 from the 2H2O). The updated equation is shown below.
The carbons and hydrogens are balanced, but the oxygens are not balanced. Currently, there are 2 oxygens on the left and 4 oxygens on the right. We are able to balance the oxygens by placing a 2 in front of the O2 (we have 2O2). We have made a modification to the left side of the reaction, so I am going to count the number of each of the 3 different elements again; on the left side of the equation, we have 1 carbon atom, 4 hydrogen atoms and 4 oxygen atoms.
The number of the 3 different elements are now balanced; we have 1 carbon atom on both sides, 4 hydrogen atoms on both sides and 4 oxygen atoms on both sides. We now have the balanced equation.
The equation below is for the reduction of iron (III) oxide using carbon.
Like the two questions before, I am going to write out the different elements under the reaction arrow and find the number of the different elements. For this reaction, the different elements are iron, oxygen and carbon. We then find out the number of the different elements in the reactants (left side of the equation) and the products (the right side of the equation).
For the reactants, there are 2 irons, 3 oxygens and 1 carbon.
For the products, there is 1 iron, 2 oxygens and 1 carbon.
From looking at the numbers, we can see that this equation is not balanced for all 3 different elements. Let’s start by balancing oxygen. At the moment, there are 3 oxygens in the reactants and 2 oxygens in the products. The only way that the number of oxygens can be balanced is to have 6 oxygens on both sides of the reaction; we want to have 6 oxygens because the lowest common multiple of 2 and 3 is 6.
In order to have 6 oxygens on the left side of the equation, we add a 2 in front of the iron oxide (2Fe2O3).
In order to have 6 oxygens on the right side of the equation, we add a 3 in front of the carbon dioxide (3CO2).
We then recount the number of the 3 different elements in the reactants and products.
For the left side of the equation, there are 4 irons, 6 oxygens and 1 carbon.
For the right, there is 1 iron, 6 oxygens and 3 carbons.
The oxygens are now balanced, but the irons and carbons are not balanced.
Let’s now balance the irons. Currently there are 4 iron atoms on the left and 1 on the right. We can balance these by placing a 4 in front of iron on the right side. As we have added in a 4 to the right, we should recount the number of the 3 different elements on the right side of the equation. When we do this, we see that there are 4 irons, 6 oxygens and 3 carbons.
The irons and oxygens are now balanced, but the carbons are not. There is only 1 carbon in the reactants and 3 carbons in the products. We can balance the carbons by placing a 3 in front of carbon. We now recount the different elements on the left side of the equation; there are now 4 irons, 6 oxygens and 3 carbons.
This equation is now balanced because we have 4 irons, 6 oxygens and 3 carbons on both sides of the equation.