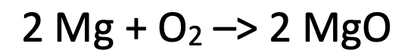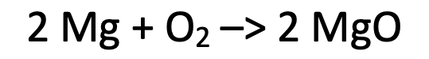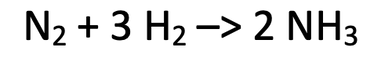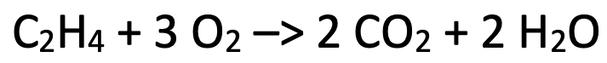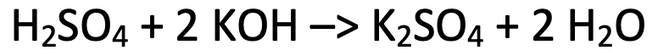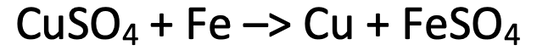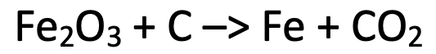Back to C3 Home
C3: Quiz 6 – Answers
C3: Quiz 6 – Answers
1)
a) A limiting reactant is a reactant that will be fully used up during a reaction and cause a reaction to stop
b) A reactant being in excess means that there is a greater quantity than necessary to complete the reaction with the limiting reactant.
2)
a) A limiting reactant is a reactant that will be fully used up during a reaction and cause a reaction to stop
b) A reactant being in excess means that there is a greater quantity than necessary to complete the reaction with the limiting reactant.
2)
3)
4)
a) 160 g
b) 64 g
5)
a) 68 g
b) 12 g
6)
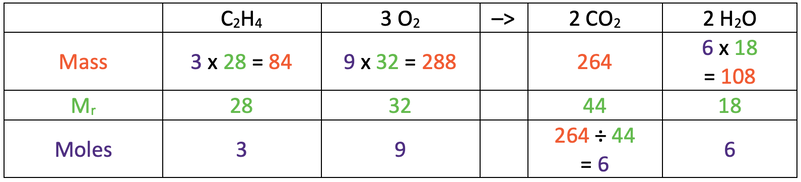
a) 84 g
b)
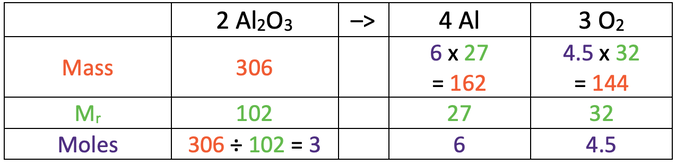
7)
a) 162 g
b) 144 g
The completed table is shown below.
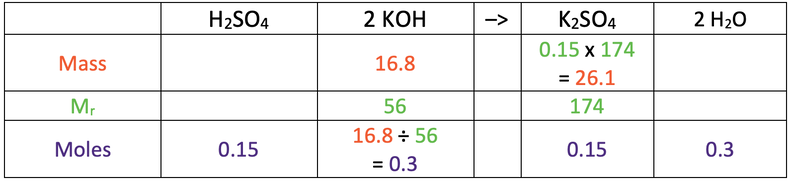
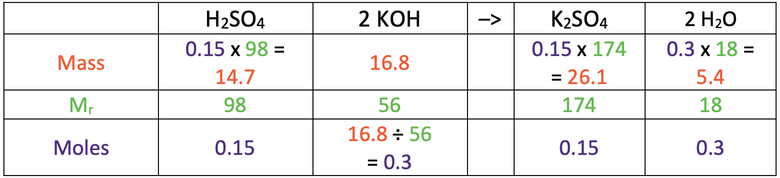
8) 26.1 g
Here are the values you would need in the table to find the answer
8) 26.1 g
Here are the values you would need in the table to find the answer
Here is the completed table.
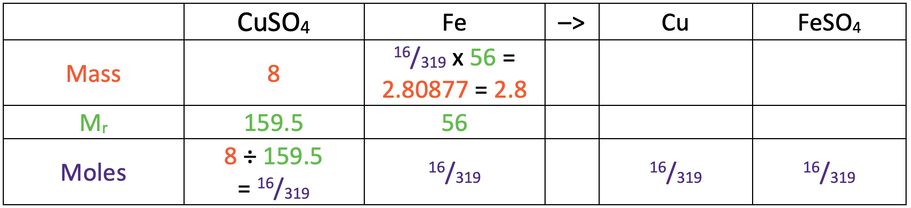
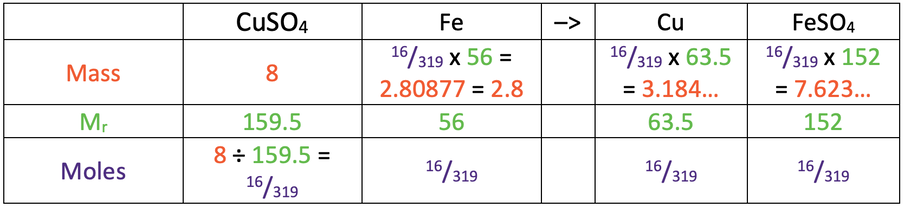
9) 2.8 g
Here are the values you would need in the table to find the answer
The completed table.
10)
a) 2 Fe2O3 + 3 C –> 4 Fe + 3 CO2
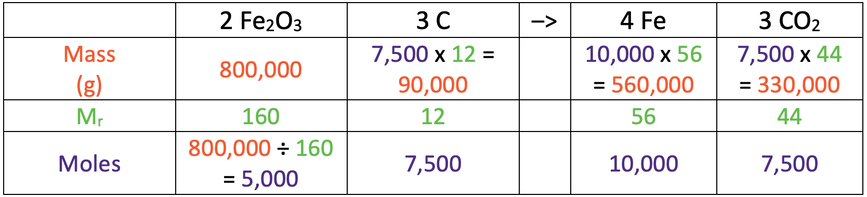
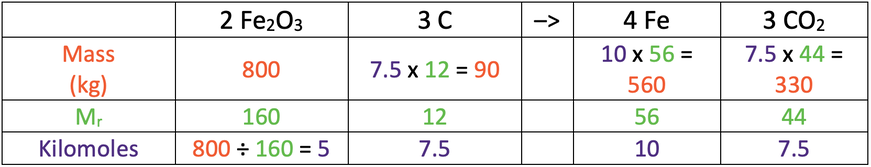
b) 560 kg
This is the table for moles.
This is the table for kilomoles.
1)
a) What does a limiting reactant mean?
b) What does a reactant being in excess mean?
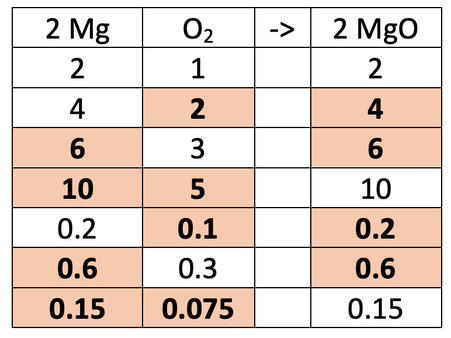
2) The equation below shows the reaction of magnesium (Mg) and oxygen (O2) to produce magnesium oxide (MgO).
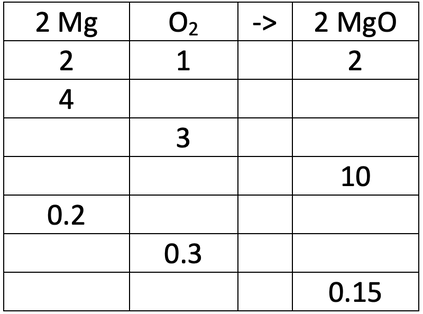
The ratio of magnesium to oxygen to magnesium oxide is 2 : 1 : 2. So, 2 moles of magnesium will react with 1 mole of oxygen to produce 2 moles of magnesium oxide. This is shown in the table below.
Fill in the gaps in the ratio table above.
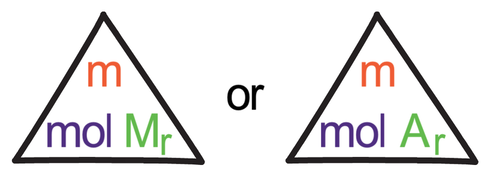
3) Write down the moles formula triangle.
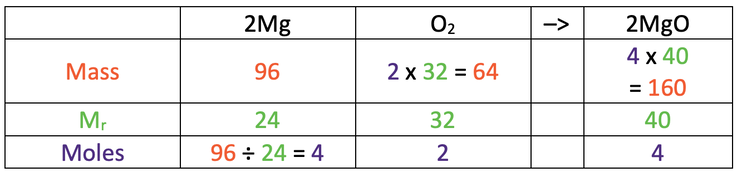
4) 96 grams of magnesium is burnt in air (oxygen) to give us magnesium oxide.
The balanced symbol equation is shown below.
3) Write down the moles formula triangle.
4) 96 grams of magnesium is burnt in air (oxygen) to give us magnesium oxide.
The balanced symbol equation is shown below.
Mg = 24, O = 16
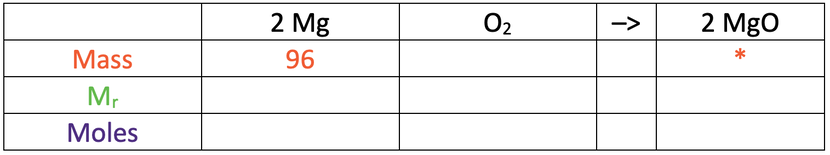
a) Calculate the mass of magnesium oxide produced.
Use the table below to help you; the star is what we are looking for.
a) Calculate the mass of magnesium oxide produced.
Use the table below to help you; the star is what we are looking for.
b) Calculate the mass of oxygen that reacts.
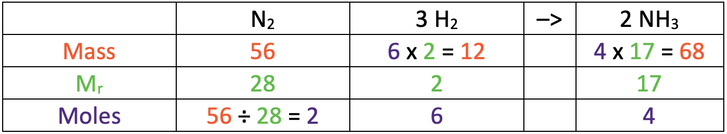
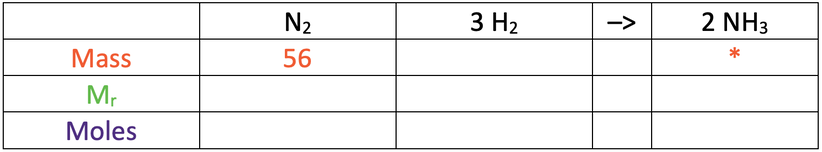
5) Nitrogen and hydrogen react to produce ammonia. The balanced equation for the reaction is shown below.
5) Nitrogen and hydrogen react to produce ammonia. The balanced equation for the reaction is shown below.
I react 56 grams of nitrogen with an excess amount of hydrogen.
N = 14, H = 1
a) Find the mass of ammonia produced.
Use the table below to help you; the star is what we are looking for.
N = 14, H = 1
a) Find the mass of ammonia produced.
Use the table below to help you; the star is what we are looking for.
b) Find the mass of hydrogen that reacts.
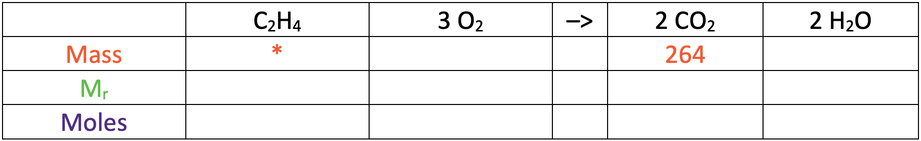
6) Ethene reacts with excess oxygen to produce carbon dioxide and water. The equation for the reaction is shown below.
6) Ethene reacts with excess oxygen to produce carbon dioxide and water. The equation for the reaction is shown below.
264 grams of carbon dioxide is produced from this reaction.
C = 12, H = 1, O = 16
a) Find the mass of ethene that was burnt.
Use the table below to help you; the star is what we are looking for.
C = 12, H = 1, O = 16
a) Find the mass of ethene that was burnt.
Use the table below to help you; the star is what we are looking for.
b) Also, complete the table.
7) We can extract aluminium from the ore aluminium oxide by undertaking electrolysis. The balanced equation for this reaction is shown below.
7) We can extract aluminium from the ore aluminium oxide by undertaking electrolysis. The balanced equation for this reaction is shown below.
a) Calculate the mass of aluminium that can be obtained when 306 grams of aluminium oxide is electrolysed.
Al = 27, O = 16
b) Calculate the mass of oxygen produced when 306 grams of aluminium oxide is electrolysed.
8) Sulfuric acid reacts with potassium hydroxide to produce potassium sulfate and water. The balanced symbol equation for this reaction is shown below.
Al = 27, O = 16
b) Calculate the mass of oxygen produced when 306 grams of aluminium oxide is electrolysed.
8) Sulfuric acid reacts with potassium hydroxide to produce potassium sulfate and water. The balanced symbol equation for this reaction is shown below.
Calculate the mass of potassium sulfate produced when 16.8 grams of potassium hydroxide reacts with excess sulfuric acid.
H = 1, S = 32, O = 16, K = 39
9) We can obtain copper from copper sulfate by displacing it with iron. The equation for this reaction is shown below.
H = 1, S = 32, O = 16, K = 39
9) We can obtain copper from copper sulfate by displacing it with iron. The equation for this reaction is shown below.
Calculate the minimum mass of iron that is required to displace copper from 8 grams of copper sulfate. Give your answer to 2 significant figures.
Cu = 63.5, S = 32, O = 16, Fe = 56
10) We can extract iron from the ore iron (III) oxide by using carbon. The unbalanced equation for this is.
Cu = 63.5, S = 32, O = 16, Fe = 56
10) We can extract iron from the ore iron (III) oxide by using carbon. The unbalanced equation for this is.
a) Balance the chemical equation above.
b) A firm reacts 800 kg of iron (III) oxide with excess carbon. Calculate the mass of iron that the firm can expect to produce; give your answer in kilograms.
Fe = 56, O = 16, C = 12
Note: you can use kilomoles or moles
b) A firm reacts 800 kg of iron (III) oxide with excess carbon. Calculate the mass of iron that the firm can expect to produce; give your answer in kilograms.
Fe = 56, O = 16, C = 12
Note: you can use kilomoles or moles