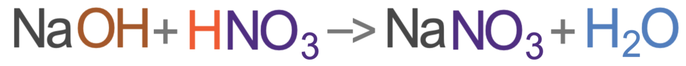C4 C) Reactions of Acids
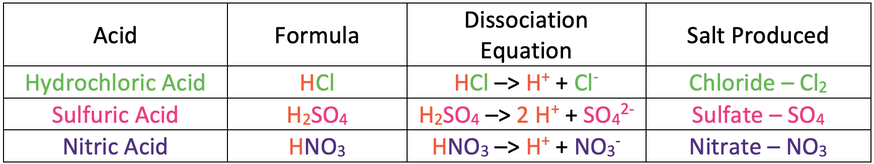
We are going to be looking at the different types of reactions with acids in this section. All of the reactions that we will look at will produce a salt. Before we look at the reactions, I am going to go through some common acids and the salts that they produce. The names, formulas, dissociation equations and salts that the acids produce are shown in the table below.
Metals & Acids
The reaction of a metal and an acid produces a salt and hydrogen. The word equation is shown below.
The hydrogen that is produced by this reaction will bubble through the acid. We can test for the presence of hydrogen by placing a lit splint where the gas is produced; if we hear a squeaky pop, it tells us that hydrogen gas is produced.
Let’s now write the word and chemical equations for the reaction of magnesium (Mg) with hydrochloric acid (HCl), which will produce a salt and hydrogen. All salts from hydrochloric acid are chlorides, and the metal involved is magnesium. This means that the salt produced will be magnesium chloride. The word and chemical equations are shown below.
I am now going to write the word and chemical equations for the reaction of iron (Fe) with sulfuric acid (H2SO4), which will also produce a salt and hydrogen. All salts from sulfuric acid sulfates, and the metal involved is iron. This means that the salt produced will be iron sulfate. The word and chemical equations are shown below.
Bases
There are three different types of bases, which are metal oxides, metal hydroxides and metal carbonates. We are now going to have a look at the reactions of these three different bases with acids.
Metal Oxide
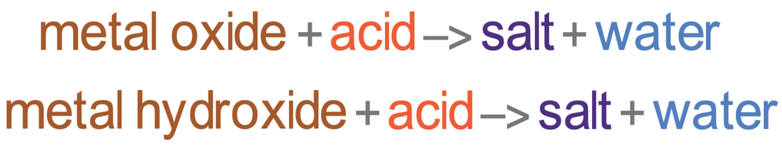
Metal oxides contain a metal and oxygen. The oxygen will have a charge of 2- (O2-). The reaction between metal oxides and acids produce a salt and water. The word equation is shown below.
Let’s now write the word and chemical equations for the reaction of copper oxide (CuO) with hydrochloric acid (HCl), which will produce a salt and water. All salts from hydrochloric acid are chlorides, and the metal involved is copper. This means that the salt produced will be copper chloride. The word and chemical equations are shown below.
I am now going to write the word and chemical equations for the reaction of potassium oxide (K2O) with sulfuric acid (H2SO4), which will also produce a salt and water. All salts from sulfuric acid are sulfates, and the metal involved is potassium. This means that the salt produced will be potassium sulfate. The word and chemical equations are shown below.
Metal Hydroxide
Metal hydroxides are made out of a metal ion and a hydroxide ion. The hydroxide ion has a charge of -1 (OH-). The reaction between metal hydroxides and acids produce a salt and water. The word equation is shown below.
Let’s now write the word and chemical equations for the reaction of sodium hydroxide (NaOH) with nitric acid (HNO3), which will produce a salt and water. All salts from nitric acid are nitrates, and the metal involved is sodium. This means that the salt produced will be sodium nitrate. The word and chemical equations are shown below.
Metal Carbonate
Metal carbonates are made out of a metal ion and a carbonate ion. The carbonate ion has a charge of negative 2 (CO32-). The reaction of a metal carbonate and an acid produces a salt, water and carbon dioxide. The word equation is shown below.
We can test for the presence of carbon dioxide by bubbling the gas produced through limewater, which is a colourless solution. If carbon dioxide is present, the limewater will turn cloudy – a precipitate will form in the limewater.
Let’s now write the word and chemical equations for the reaction of calcium carbonate (CaCO3) with sulfuric acid (H2SO4), which will produce a salt, water and carbon dioxide. All salts from sulfuric acid are sulfates, and the metal involved is calcium. This means that the salt produced will be calcium sulfate. The word and chemical equations are shown below.
I am now going to write the word and chemical equations for the reaction of copper carbonate (CuCO3) with hydrochloric acid (HCl), which will also produce a salt, water and carbon dioxide. All salts from hydrochloric acid are chlorides, and the metal involved is copper. This means that the salt produced will be copper chloride. The word and chemical equations are shown below.
A Summary
I am now going to go through a summary of the different reactions with acids. All of these reactions produce a salt.
The reaction of a metal and an acid produces a salt and hydrogen.
The reaction of a metal oxide or a metal hydroxide with an acid produces a salt and water. Water (H2O) is produced rather than just hydrogen (H2) because metal oxides and metal hydroxides contain oxygen.
The reaction of a metal carbonate and an acid produces a salt, water and carbon dioxide. Carbon dioxide (CO2) is produced in addition to a salt and water because the metal carbonates contain carbon and oxygen.