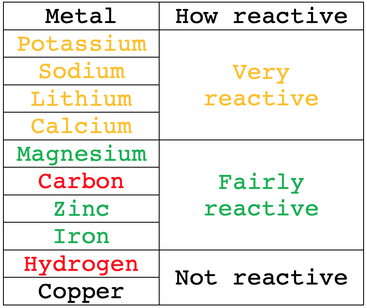
C4 G) Displacement Reactions
Example 1 – Reaction
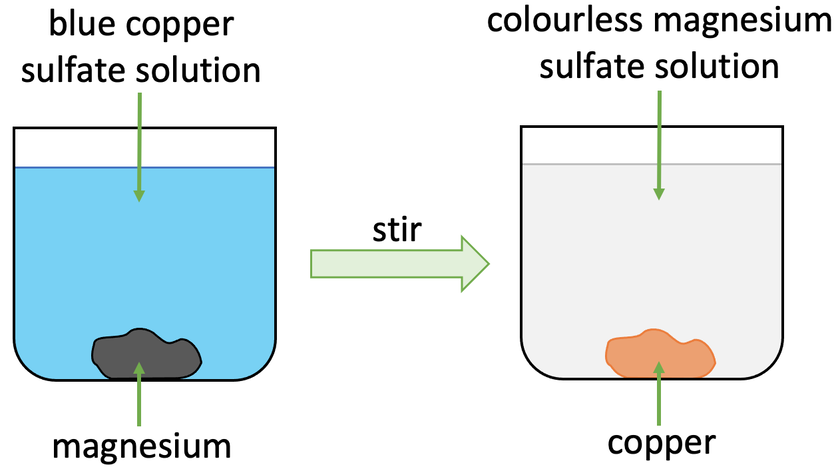
A more reactive metal will displace a less reactive metal from its compound. For example, let’s look at what would happen if we added magnesium to a copper sulfate solution (CuSO4).
Magnesium is more reactive than copper. This means that magnesium will displace the copper in copper sulfate resulting in magnesium sulfate (MgSO4) and copper. The magnesium sulfate will be in the solution (aqueous) and the copper will be solid. The word and chemical equations are shown below:
Before this reaction takes place, the copper sulfate solution would be blue. We then add some magnesium to the copper sulfate solution, which results in the reaction starting. The magnesium that we add will displace the copper in copper sulfate resulting in magnesium sulfate (aqueous) and copper (solid). The solution in the beaker will become colourless as magnesium sulfate solution is colourless. The copper will sit at the bottom of the beaker/ container.
The same sort of reaction would take place if we added zinc instead of magnesium to the copper sulfate solution. Zinc is more reactive than copper, which means that the zinc would displace copper in copper sulfate to produce zinc sulfate and copper.
Example 2 – No Reaction
If we tried to react a less reactive metal with a more reactive metal compound, no reaction would take place. This is because the less reactive metal will be unable to displace the more reactive metal. For example, no reaction would take place if we added copper to a solution of calcium sulfate. This is because copper is less reactive than calcium, which means that copper will be unable to displace the calcium in calcium sulfate.