Back to C4 Home
C4 I) Extracting Metals – Carbon
C4 I) Extracting Metals – Carbon
Metals tend to react with oxygen to produce metal oxides. These metal oxides are found in rocks in the ground that are known as ores – an ore is a rock that contains metal compounds in sufficient quantities to make it worthwhile extracting the metal.
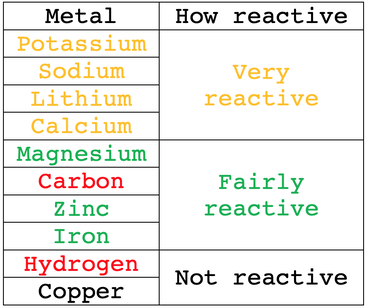
The method that we use to extract the metal from its ore/ metal oxide depends on how reactive the metal is. The reactivity series for the main metals is shown below with the most reactive metals at the top and the least reactive metals at the bottom.
The method that we use to extract the metal from its ore/ metal oxide depends on how reactive the metal is. The reactivity series for the main metals is shown below with the most reactive metals at the top and the least reactive metals at the bottom.
Carbon and hydrogen are not metals, but they are also included in the reactivity series.
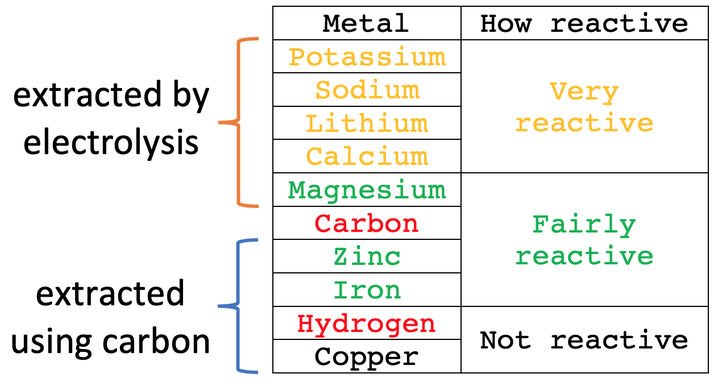
Metals that are more reactive than carbon are extracted from their ores by using electrolysis (using electricity). Metals that are less reactive than carbon are extracted from their ores by using carbon. We can extract all metals from their ores by using electrolysis, but electrolysis uses vast quantities of electricity and is therefore very expensive; electrolysis is considerably more expensive than extracting by using carbon. Therefore, if we can extract a metal from its ore by using carbon, we use carbon to extract the metal from its ore – however, we can only use carbon for metals that are less reactive than carbon.
Metals that are more reactive than carbon are extracted from their ores by using electrolysis (using electricity). Metals that are less reactive than carbon are extracted from their ores by using carbon. We can extract all metals from their ores by using electrolysis, but electrolysis uses vast quantities of electricity and is therefore very expensive; electrolysis is considerably more expensive than extracting by using carbon. Therefore, if we can extract a metal from its ore by using carbon, we use carbon to extract the metal from its ore – however, we can only use carbon for metals that are less reactive than carbon.
In this section, we are going to look at the extraction of metals from their ores by using carbon. We will look at electrolysis in the next couple of sections.
Zinc Oxide
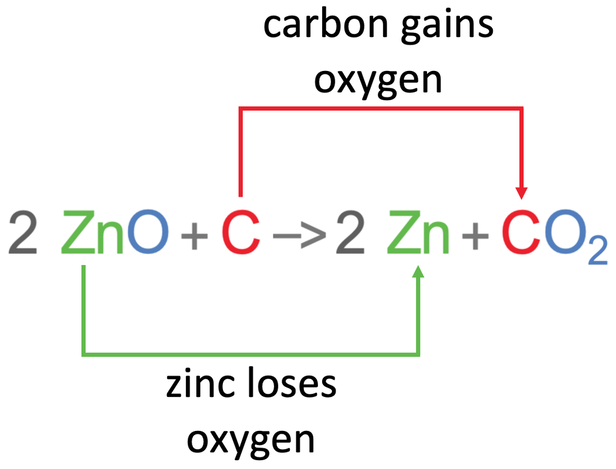
Zinc is less reactive than carbon, which means that zinc can be extracted from zinc oxide by using carbon. When zinc oxide and carbon react, the more reactive carbon takes the oxygen off of the less reactive zinc. This results in the products of the reaction being pure zinc and carbon dioxide. The word and chemical equations for this reaction are shown below.
Zinc is less reactive than carbon, which means that zinc can be extracted from zinc oxide by using carbon. When zinc oxide and carbon react, the more reactive carbon takes the oxygen off of the less reactive zinc. This results in the products of the reaction being pure zinc and carbon dioxide. The word and chemical equations for this reaction are shown below.
We have now successfully extracted zinc from zinc oxide.
Oxidation and Reduction – Oxygen
Oxidation is the gain of oxygen, and reduction is the loss of oxygen (it is worth getting this down on a revision card). For the above reaction, zinc lost oxygen which means that zinc has been reduced. The carbon gained oxygen, which means that carbon has been oxidised.
Oxidation and Reduction – Oxygen
Oxidation is the gain of oxygen, and reduction is the loss of oxygen (it is worth getting this down on a revision card). For the above reaction, zinc lost oxygen which means that zinc has been reduced. The carbon gained oxygen, which means that carbon has been oxidised.
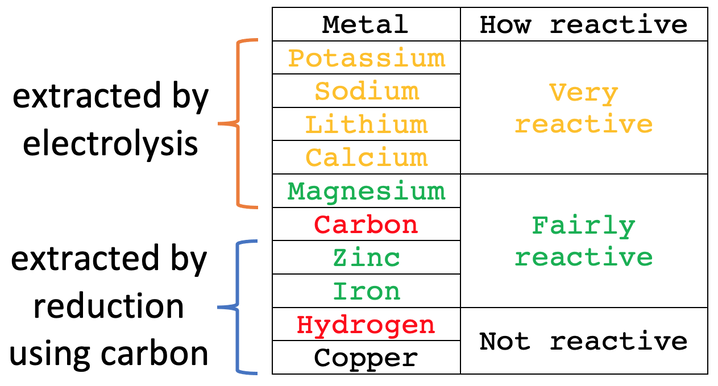
The metal that we are extracting is always the substance that is reduced as it loses oxygen, and the carbon is always oxidised as it gains oxygen. As the metal that we are extracting is reduced, we can modify our reactivity series table and say that metals that are less reactive than carbon are “extracted by reduction using carbon”. I have added this modification to the reactivity series.
Iron (III) Oxide
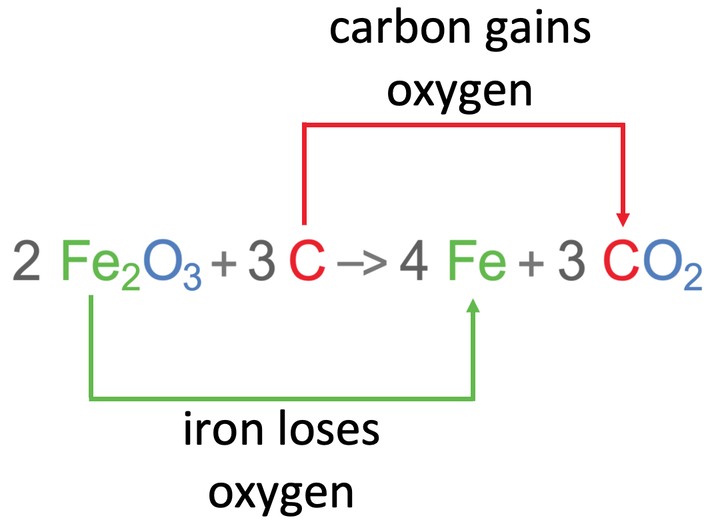
We are now going to have a look at the extraction of iron (III) oxide which is Fe2O3. According to the reactivity series, iron is less reactive than carbon. This means that iron can be extracted from iron (III) oxide by reduction using carbon. The word and chemical equations for this reaction are shown below.
In this reaction, iron lost oxygen, which means that iron has been reduced. Also, the carbon gained oxygen, which means that carbon has been oxidised.
This follows the rule because the metal that was being extracted was reduced – the iron was reduced as it lost oxygen. And the carbon was oxidised as it gained oxygen.