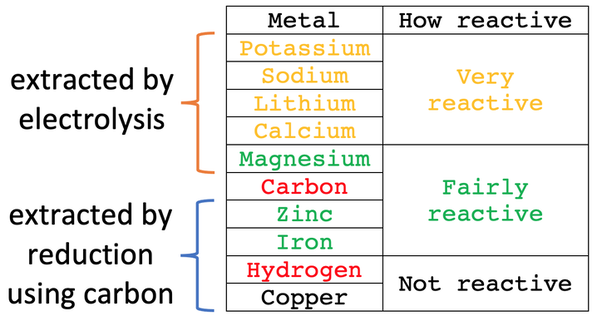
C4 J) Electrolysis – Part 1
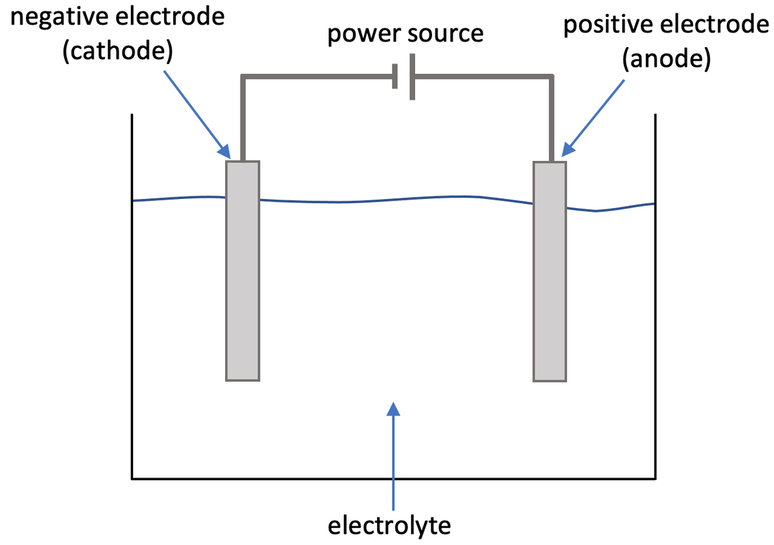
There are two electrodes in electrolysis; a positive and a negative electrode. The positive electrode is known as the anode, and the negative electrode is known as the cathode. The electrodes are usually made of graphite, which is a giant covalent structure of carbon atoms. The electrodes are made of graphite because graphite has a very high melting point and is a very good conductor of electricity.
The power supply for electrolysis produces a direct current (DC) because we want the electrons to flow around in only one direction (we do not want the power supply to produce an alternating current (AC)).
The positive metal ions in the ionic compound are attracted to the cathode (negative electrode) because opposite charges attract. The positive metal ions at the cathode gain electrons – they are reduced because they gain electrons (remember OIL RIG; Oxidation Is Loss, Reduction Is Gain).
The negative non-metal ions in the ionic compound are attracted to the anode (positive electrode) because opposite charges attract. The negative non-metal ions at the anode lose electrons – they are oxidised because they lose electrons (OIL RIG; Oxidation Is Loss, Reduction Is Gain).
At both of the electrodes, the ions gain or lose electrons resulting in them losing their charge and being discharged from the electrolyte; they may sit at the bottom of the electrolyte tank or become a gas and enter the air in the lab.
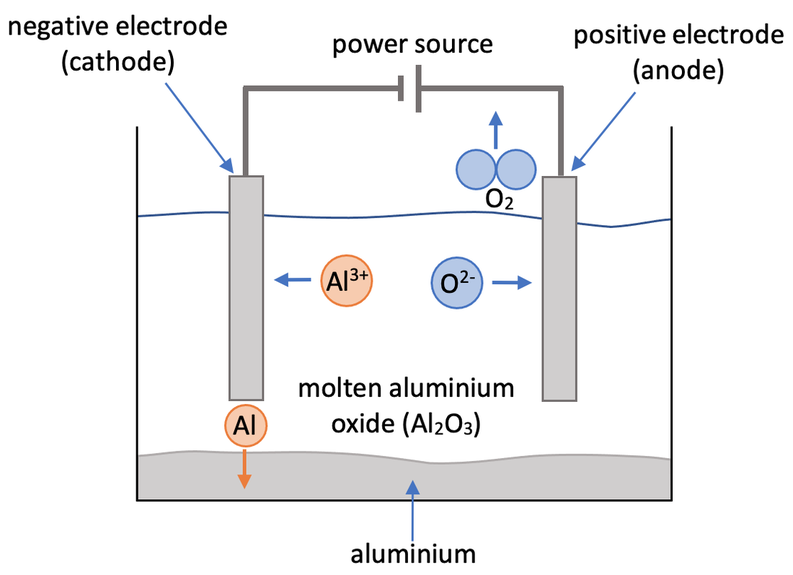
We are now going to have a look at using electrolysis to obtain pure aluminium. We obtain aluminium from an ore called bauxite, which contains aluminium oxide (Al2O3). Aluminium oxide is made out of positive aluminium ions that have a charge of 3+ (Al3+), and negative oxide ions that have a charge of 2- (O2-).
Aluminium oxide is insoluble in water (cannot be dissolved in water). Aluminium oxide has an extremely high melting point, which means that it would require vast quantities of energy to melt it, thus making the electrolysis process extremely expensive. Therefore, we dissolve powdered aluminium oxide in molten cryolite to lower the melting point, which requires less energy, thus reducing the cost of the electrolysis process. The setup of the experiment is shown below.
Aluminium Ions
The positive aluminium ions (Al3+) are attracted to the negative electrode (cathode) where they gain electrons. The aluminium ions have a charge of 3+, which means that each aluminium ion picks up 3 electrons at the cathode. The half equation for this is shown below.
After each of the positive aluminium ions have picked up 3 electrons, they will have a neutral charge and be discharged from the electrolyte – they sink to the bottom of the electrolyte tank. Also, as the positive aluminium ions have gained electrons, aluminium has been reduced.
Oxygen Ions
The negative oxide ions (O2-) are attracted to the positive electrode (anode) where they lose electrons. The oxide ions have a charge of 2-, which means that each oxide ion loses two electrons at the anode. The oxygens that have lost electrons combine to form O2 molecules. The half equation for this is shown below.
The oxygen gas produced (O2) will have a neutral charge and be discharged from the electrolyte – the oxygen gas will leave the electrolyte tank and enter the air in the lab. Also, as the negative oxygen ions have lost electrons, oxygen has been oxidised.
Note
Both of the half equations must balance within each of the half equations. But, the two half equations do not need to balance with each other. For example, the first half equation for aluminium involved 3 electrons and the second half equation for oxygen involved 4 electrons – it does not matter that the number of electrons in the two half equations are not the same; it only matters that each of the half equations balances on their own.
The Overall Reaction
The overall word and chemical equations for the extraction of aluminium from aluminium oxide are shown below.
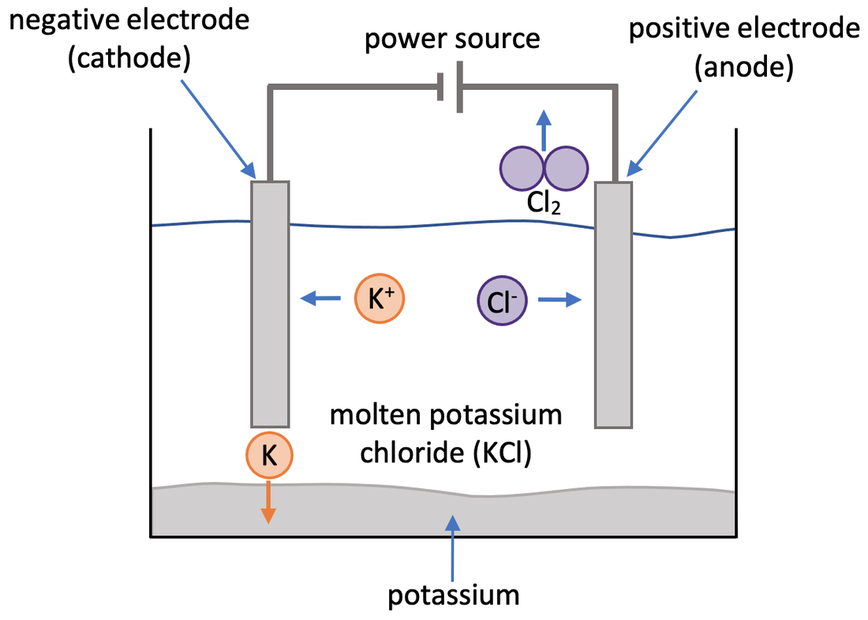
We are now going to look at the electrolysis of molten potassium chloride (KCl). Potassium chloride has positive potassium ions (K+) and negative chloride ions (Cl-). The setup of the electrolysis of molten potassium chloride is shown below.
Potassium Ions
The positive potassium ions (K+) are attracted to the negative electrode (cathode) where they gain 1 electron to become neutrally charged. The half equation for this is shown below.
After each of the positive potassium ions have picked up 1 electron, they will have a neutral charge and be discharged from the electrolyte – they sink to the bottom of the electrolyte tank. Also, as the positive potassium ions have gained 1 electron, potassium has been reduced.
Chloride Ions
The negative chloride ions (Cl-) are attracted to the positive electrode (anode) where they lose 1 electron to become neutrally charged. The chloride ions that have lost 1 electron combine to form chlorine gas (Cl2). The half equation for this is shown below.
The chlorine gas produced (Cl2) will have a neutral charge and be discharged from the electrolyte – the chlorine gas will leave the electrolyte tank and enter the air in the lab. Also, as the negative chloride ions have lost 1 electron, chlorine has been oxidised. We can test for the presence of chlorine by holding damp blue litmus paper where the chlorine gas is produced; if chlorine is present, the chlorine will bleach the damp blue litmus paper turning it white (the litmus paper may turn red before it turns white because the solution of chlorine is acidic).